金属注射成不锈钢17-4PH: 制程、性质与最佳的颗粒
2018/8/9 18:51:47
新材料在线
154754
耀德讲堂长篇专案翻译:邱耀弘博士/赵育德硕士
This paper was populated on page 49~76 Vol.12 No.2, June 2018, Powder Injection Molding International. 本文发表于国际粉末注射成形期刊 2018 年6月份第十二卷第二期第49~76页

Beginning of this article 本文开始
注意翻译按照有关英式英文内容已更改为美式英文!蓝色字体为译者补充说明。红字的提醒为译者认为读者们要注意是在过去的观念上有所忽略或是不一样的地方,特别强调。绿字为实战补充,根据文章内容提出有关补充。
In the Metal Injection Molding industry, 17-4 PH stainless steel is one of the most popular materials thanks to its combination of strength, hardness and corrosion resistance. As a result of its success in MIM, it is also attracting interest for use in the growing number of ‘MIM-like’ Additive Manufacturing processes, including binder jetting and feedstock extrusion. Despite the alloy’s popularity, there remain limited data on the final properties that can be expected, as well as data relating to dimensional control and the impact of Hot Iso-static Pressing. In the following article, Prof Randall German highlights best practice in the de-binding and sintering of 17-4 PH, as well as presenting in-depth analysis of published data. 在金属注射成形产业中,17-4PH不锈钢由于其强度、硬度和耐腐蚀性的结合而成为最流行的材料之一。由于其在MIM的成功表现,它吸引了越来越多的“类MIM”增材制造工艺,包括粘结剂喷射和喂料挤出的。尽管此合金很受欢迎,但其最终的性能却只有很有限的资料可以参考,有关尺寸控制和热等静压的影响的资料都甚少。在本文章中,German教授强调了17-4 PH的脱脂、烧结的最佳实践作业,并对已发表的文献进行了深入的归类分析。
Sintering is a means to fabricate complex, high-performance stainless steel components from powders via injection molding, die compaction, binder jetting, paste extrusion and other binder-assisted routes. The sintering behavior of 17-4 PH stainless steel depends on particle size, peak temperature, heating rate, atmosphere and hold time. MIM制品的烧结是通过注射成型、模内压缩、粘结剂喷流、喂料膏体挤出和其他粘结剂辅助条件,把高性能不锈钢从粉末制备做成复杂部件的方法。17-4PH不锈钢的烧结行为也是取决于粉末颗粒大小、峰值温度、加热速率、气氛和保温时间等。
Complications arise from alloy composition variations and retained carbon and oxygen. Some binders add carbon during burnout and some powders carry high oxygen content. Carbon from the powder or binder, oxygen from the powder or atmosphere and nitrogen from the process atmosphere influence the micro-structural phases, sintering behavior and final properties. Carbon and nitrogen stabilize the face-centered cubic austenite phase, slowing sintering. On the other hand, body-centered cubic delta ferrite increases the sintering rate. Martensite forms on cooling from the sintering temperature and precipitation reactions induce a high hardness and strength. However, retained delta-ferrite reduces strength. 烧结件的问题并发症发生于合金成分变化和保留的碳和氧元素,一部分粘结剂在烧毁过程残留变成碳增量,一些粉末则携带高氧含量。粉末中固有或粘结剂残留造成的碳、粉末中固有或气氛中的氧和工艺使用气氛中的氮,都将影响产品的微观结构相、烧结行为和最终性能。碳和氮稳定了面心立方奥氏体相,减缓烧结;另一方面,体心立方δ-铁素体出现会增加烧结速率。在烧结温度和沉淀反应的冷却过程中,马氏体形成高硬度和高强度。然而,保留δ-铁素体降低了烧结件的强度。
An added difficulty comes from the segregation of alloying ingredients during sintering, leading to heterogeneous microstructures with reduced properties. Doping additions, such as boron, reduce the sintering temperature and improve properties. Manipulation of densification and phases leads to competitive properties. However, in spite of good strength and hardness, impact toughness is low. Considerable data are collected on how 17-4 PH stainless steel sinters and typical mechanical sintered and heat treated properties, with comments on corrosion, wear and biocompatibility. 另一个困难来自烧结过程中合金的成分偏析,导致异质性的微观结构并降低产品的性能。掺杂如硼,则可降低烧结温度和改善性能。致密化和相的操作导致了两者互相的竞争性,然而,产品尽管具有良好的强度和硬度,但冲击韧性却因此降低。本文收集了17-4PH不锈钢烧结件其典型的烧结后机械和热处理后性能的大量资料,并对抗腐蚀、磨损和生物相容性进行了评价。
在产品交货过成一定要注意到,原材料添加任何标准材料以外的元素和添加剂,都必须向客户沟通并取得”免责权”,否则当产品实际应用于商品的时候,一但出现问题并进行追究赔偿时,供应商和客户都会蒙受巨大的损失,在论文上的实验以添加额外的元素而超出国际规范标准时,结果将是没有办法被国际协会认证材料标准所保护,要非常注意。
An introduction to 17-4 PH沉淀硬化不锈钢的简介
Stainless steels are ferrous alloys containing at least 12 wt% chromium. They arose in the early 1900s, with the first patents in the 1910-1919 time frame. These alloys are categorized according to their main phase; austenitic, ferrite, martensitic and semi-austenitic. Some of the alloys are responsive to precipitation hardening. The precipitation hardened martensitic stainless steels range from 12 to 17 wt.% chromium, 4 to 8 wt.% nickel, and 0 to 4 wt.% copper, with possible additions of molybdenum, silicon, manganese, titanium and niobium. Of these, 17-4 PH is a popular variant, consisting of iron alloyed as outlined in Table 1 [1, 2]. This alloy also carries identifications such as UNS S17400, AISI 630, ASTM A564, MIM-17-4PH and AMS 5643. 能称之为不锈钢是含至少12wt%铬的铁合金,它们出现于1900年代早期,在1910年至1919年的时间被申请了第一个专利(英国谢菲尔德大学的着名冶金科学家亨利·布雷尔利Harry Brearley)。这些不生锈的合金按其主要相分类:奥氏体、铁素体、马氏体和半奥氏体,以及一些具有沉淀硬化的能力-沉淀硬化的马氏体不锈钢,其铬含量范围从12~17wt%,4~8wt%的镍,和0~4wt%的铜,与可能的添加钼、硅(硅)、锰、钛和铌。所有的不锈钢中,17-4PH是一种受欢迎的变体不锈钢,由表1 [1, 2]中概述的元素成分组成。该合金还具有其他标识称号,如UNS S17400,AISI 630,ASTM A564,MIM-17-4PH和AMS 5643。
Table 1 Nominal composition for wrought and powder alloys in wt.% (allowed Cr, Ni, and Cu variations are ± 1 wt.%) 不锈钢17-4PH的常规锻造(Wrought)料与粉末合金(Powder alloys)的成分重量百分比比较(允许铬、镍、和铜具有+/-1wt%误差)
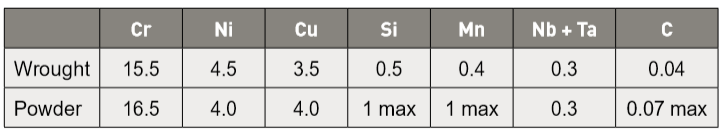
The compositional specifications do not include oxygen. However, oxygen is a significant factor in sintered products. A powder has a high initial surface area coated with oxides. Accordingly, the initial oxygen level varies with the powder production process. These oxides remove chromium from its corrosion inhibition role. High oxygen levels result in low mechanical properties and poor corrosion resistance [3, 4]. Indeed, isolation of the oxygen role in sintered 17-4 PH stainless steel is an area requiring further study. 不锈钢17-4PH的组成规格并不包括氧,然而,氧含量却是烧结件中的一个重要影响因素。粉末通常具有很多的氧化物覆盖在初始表面积,因此,初始氧的水准随粉末生产过程而变化。这些氧化物由其缓蚀作用会慢慢拿走铬的防锈能力,高含氧量导致烧结件的机械性能降低和耐腐性变差[3, 4]。事实上,烧结不锈钢17-4PH如何把所含的氧去除是一个需要进一步研究的领域。
“Applications for 17-4 PH arise from the combination of strength, hardness and corrosion resistance.” 17-4PH应用的崛起来自强度、硬度和抗腐蚀的综合需求
It is not the best stainless steel in any of these categories, but, after heat treatment, the property combination is attractive for aerospace, medical, dental, nuclear and consumer products. Depending on the composition (due to the allowed variations in Fe, Ni, Cu and Cr), the solidus temperature varies around 1405°C and the liquids temperature is near 1440°C. Because of precipitation hardening, the mechanical properties are sensitive to heat treatment. The full density elastic modulus is from 196 to 204 GPa [5]. Depending on heat treatment, the hardness reaches 43 HRC, with a yield strength from 760 to 1240 MPa, tensile strength from 1000 to 1340 MPa and fracture elongation between 8 and 14%. This high hardness makes die compaction difficult, but binder-assisted compaction, as well as injection molding, feedstock extrusion and binder jetting and related ideas, are successful. 在所有类别的不锈钢中,17-4PH虽不是最好的,但是经过热处理后其性质组合是有吸引力的,他们可应用于航空航太、医疗、牙科、核能和消费产品。根据其成分(由于铁、镍、铜和铬的允许变化),固相线温度在1405°C附近变化,并且液体温度接近1440°C。由于析出硬化,机械性能对热处理敏感。全密度的弹性模量为196~204 GPa[5],根据热处理后结果,硬度达到HRC43,屈服强度从760~1240 MPa,拉伸强度从1000~1340 MPa,断裂伸长率在8~14%之间。这种高硬度的材质使得传统压制成型困难,但可改用粘结剂来辅助粉末材料的压实,再利用注射成形将喂料挤出和粘结剂注射和相关的想法,则是成功的。
Due to fluctuations in alloy composition and microstructural phases, the theoretical density and properties change accordingly. Density for wrought material is 7.66 to 8.00 g/cm3. For powder, the average pycnometer density is 7.77 g/cm3. In the as-sintered condition, the average theoretical density is 7.74 g/ cm3 but varies with delta-ferrite content. The density increases slightly with hardening heat treatments, progressively reaching 7.86 g/cm3 at maximum hardness. These changes reflect differences in atomic size and lattice packing associated with the different crystalline phases. High temperature neutron diffraction during sintering identifies lattice constants of 0.35833 nm and 0.28583 nm for the face-centred cubic and body-centred cubic phases, corresponding to densities of 7.80 and 7.68 g/cm3 [6]. Most studies on sintered 17-4 PH ignore these density shifts. For this report, the pycnometer value measured in each study is used to calculate fractional density or porosity, or, where not reported, the theoretical density is assumed to be 7.80 g/cm3. 由于合金成分和微观结构相的变动,理论密度和机械性质相应地改变。锻造件的密度为7.66~8 g/cm3,对于粉末成型而言平均比重瓶密度为7.77 g/cm3。在烧结条件下,平均理论密度为7.74 g/cm3,但δ-铁素体含量变化,密度随硬化热处理略有增加,在最大硬度下可达到7.86 g/cm3。这些变化反映了与不同结晶相关联的原子尺寸和晶格堆积的差异。烧结过程中的高温中子衍射识别面心立方和体心立方相的晶格常数为0.35833 nm和0.28583 nm,对应于密度为7.80~7.68 g/cm3[6]。大多数烧结17-4 PH忽略了这些密度的变化。对于本报告,在每个研究中测量的比重瓶值被用来计算分数密度(指的是具有孔隙率的烧结件密度),或者在没有报导的情况下,理论密度被假定为7.80 g/cm3。
Binder and de-binding effects 粘结剂和脱脂的影响
Many binder systems can be used for shaping stainless steel powders [7-40]. Injection molding and extrusion binders commonly rely on 60% filler, 30% backbone and 10% surfactant. Paraffin wax is a common filler phase, but polyethylene glycol and polyoxymethylene are also widely used. Backbone polymers are more varied, but favor polypropylene, polyethylene, ethylene vinyl acetate, or polymethyl methacrylate. Stearic acid is the most common surfactant/ lubricant/plasticiser, but palm oil, beeswax, glycerin and similar waxy or oily molecules are also in use. Binder ingredients that produce a carbon residue, such as cellulose or polystyrene, are generally avoided, since carbon has profound effects on sintering and sintered properties. Generally, simple, small and inexpensive binder ingredients are most successful. Additive Manufacturing relies on these same binders for extrusion, but also takes on variants such as sucrose, acrylic, latex, starch or other polymers sprayed onto the powder bed during the build process. 许多粘结剂系统可用于不锈钢粉末注射成形[7-40]。注射成形和挤出粘结剂通常依赖60wt%填料,30wt%的主链和10wt%的表面活性剂。其中,石蜡是一种常见的填充剂,但聚乙二醇和聚甲醛也被广泛使用。骨架聚合物变化更大,但有利于聚丙烯、聚乙烯、乙烯-乙酸乙烯酯或聚甲基丙烯酸甲酯。硬脂酸是最常用的表面活性剂/润滑剂/增塑剂,但棕榈油、蜂蜡、甘油和类似的蜡状或油性分子也被使用中。通常会避免产生碳残留物的粘结剂成分,如纤维素或聚苯乙烯,因为碳对烧结和烧结性能有深远的影响。一般来说,简单、小、便宜的粘结剂成分是最成功的。增材制造依赖于这些相同的粘结剂用于挤出,但也需要在生产过程中喷涂到粉末床上的蔗糖、丙烯酸类、胶乳、淀粉或其它聚合物的改性体。
增材制造已经和MIM技术有密不可分的关系,主要在于喂料技术把粘结剂和粉末结合一起的方式、然后又进行脱脂,并使产品烧结致密材为高性能的零件。
De-binding has some influence on properties, especially if the final oxygen or carbon levels are increased. The common first stage de-binding routes are depolymerisation or solvent extraction. For example, paraffin wax dissolves in heptane and polyethylene glycol dissolves in hot water [41]. Catalytic extraction of polyoxymethylene uses fuming nitric acid in the first stage of de-binding [42, 43]. Subsequently, the remaining backbone binder is extracted by pyrolysis during heating to the sintering temperature. Comparative tests with different binders show that 10% sintered property loss occurs in systems retaining more oxygen (0.23% oxygen versus 0.02% oxygen) [19]. Moreover, corrosion resistance is degraded by residual oxygen [4]. 脱脂对产品性能有一定的影响,特别是左右烧结件最终的氧或碳含量增加。常见的第一阶段脱脂路线是降解或溶剂萃取,例如,石蜡溶解在庚烷中,聚乙二醇溶解在热水中[41],催化萃取聚甲醛在脱脂第一阶段使用发烟硝酸[42, 43 ](2017年后中国的深圳星特烁公司发展出草酸脱脂技术更为先进,是一个MIM产业重要的技术里程碑)。随后,第二阶段再加热到烧结温度下,通过前段的加热降解剩余的骨架粘结剂。不同粘结剂的对比试验表明,在保留更多氧气(0.23%氧与0.02%氧)的系统中发生了至少10%的烧结性能损失[19]。此外,耐腐蚀性被残余氧而降低[4]。
Thermal de-binding is often performed in hydrogen or hydrogen nitrogen, but lower temperature air burnout is successful [17, 44]. Optimal binder removal incorporates holds during heating at 600 and 1000°C, using hydrogen [45]. If water atomized powder is added to gas atomized powder, the increase in oxygen from the water atomized powder helps to reduce residual carbon. The oxides react with carbon to form CO or CO2 vapor. Likewise, graphite additions, in roughly equal mass to the starting oxygen content, are effective in oxygen removal during sintering. For example, in MIM, starting with 0.2% O and 0.01% C results in 8% delta-ferrite, 0.03% oxygen and 0.015% C. Otherwise, residual carbon stabilizes austenite during sintering, resulting in slower sintering; however, with typical hold times at the peak temperature, the slower sintering rate becomes meaningless [46-48]. 热脱脂通常在氢或氢氮中进行,但可在较低温度的空气环境中燃尽粘结剂是成功的[17, 44]。在600~1000℃加热时,使用氢气可以获得最佳的粘结剂去除结果[45]。如果水雾化粉末加入到气体雾化粉末中,水雾化粉末中的氧的增加有助于减少残余碳,氧化物中的氧与金属中的碳反应形成CO或CO2蒸气。同样,与起始氧含量大致相等的石墨添加剂对烧结过程中的氧去除是有效的。例如,在MIM制程,从0.2% O和0.01% C添加在8%δ-铁素体氧体,得到最后产品是0.03%氧和0.015%C。否则,残余碳将会在烧结过程中稳定奥氏体,导致较慢的烧结;然而,在峰值温度下典型的保温时间,较慢的烧结速率变成毫无意义[46-48]。
Powder characteristics粉末特性
Three powder approaches are applied to sintered 17-4 PH stainless steel; prealloy powder, mixed powder and master alloy powder. Prealloyed implies that each particle is a microcasting with the same composition. Mixed powder implies a combination of iron, chromium, nickel and other particles, each being a single element [49]. Master alloy is a hybrid where small carbonyl iron powder is mixed with 33% alloy powder. The alloy powder has concentrated additives (51% Cr, 12% Cu, 12% Ni, 24% Fe, 0.7% Nb, with traces of Si and Mn). A few variants use tumbled or milled powders [50-52]. Prealloy powder is favored for performance, while the master alloy is favored for low cost. However, prolonged sintering is required to deliver competitive properties from mixed elemental powders. 17-4PH不锈钢的粉末获得可以透过以下方式产出:预合金粉、元素粉混合以及母合金粉末三种。预合金粉末意味着每个颗粒是具有相同成分的微小铸件;元素粉混合意味着铁、铬、镍和其他元素都以颗粒的组合,每个颗粒都是单一元素[49],母合金是一种以羰基铁粉(纯铁)与至少33%预合金粉末混合而成的混合体,此母合金用的预合金粉末有浓度很高的添加剂元素(51% Cr,12% Cu,12% Ni,24% Fe,0.7% Nb,并有Si和Mn的痕迹)。一些调整体可以使用滚筒研磨或碾磨的粉末[50-52],预合金粉末有利于性能;而母合金粉末则是低成本的,但需要延长烧结的时间,以使混合元素粉末能扩散合金化得到与预合金粉末的竞争性能。
Prealloyed powders are gas or water atomized. Because of molten metal exposure to high pressure water, the oxygen content is higher in water atomized powder. Gas atomized powder is spherical. If adhering small satellite particles are avoided, then gas atomized powder delivers a desirable high packing density and good flow properties. The less spherical, lower packing density water atomized powder starts at a lower packing density. Fig. 1 shows micrographs of typical gas and water atomized powders with a nominal particle size of 20 µm. For injection molding feedstock, the solids loading might be 62.5 vol. % for gas atomized powder and 55 vol. % for water atomized powder. Similar loadings are anticipated for extrusion Additive Manufacturing. The lower solid loading implies that more sintering densification is needed for the water atomized powder [45, 51, 53]. 预合金粉末得到的方法是以气体或水进行雾化的,如采用水雾化法,由于金属暴露于高压水中,粉末中的氧含量较高、形状较不规则;气体雾化粉末则为球形,如果能够避免附着小的卫星颗粒,则气体雾化粉可以末提供理想的高堆积密度和良好的流动性能。低球形度、低填充密度的水雾化粉末以较低的填充密度开始。图1示出了典型的气体和水雾化粉末的显微照片,其标称粒径为20μm。对于注射成形用的起始原料,就固体装量而言,气体雾化粉末可以高达的62.5vol%而水雾化粉末仅达55vol%。类似的装载量也用于挤出型增材制造(Fused Deposition Molding, FDM熔融沉积成形技术)。较低的固体负荷意味着水雾化粉末需要更多的烧结时间或温度来得到致密化[45, 51, 53]
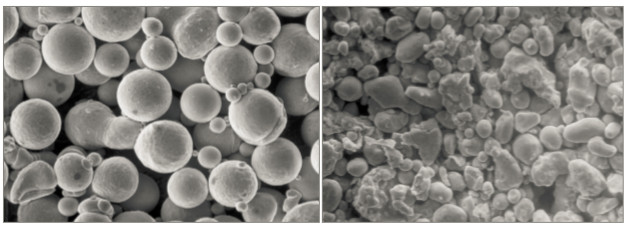
Fig. 1 Scanning electron micrographs of (a) spherical gas atomized and (b) ligament water atomized powders. The larger particles are nominally 20 µm in diameter 扫描式电子显微镜(SEM)照片(a)球形状的气体雾化;(b)韧带水雾化粉末,较大的颗粒在直径为20μm。
The typical 17-4 PH stainless steel powder has a median particle size of 10 to 12 µm, with a tap density of 4.3 g/cm3. Table 2 gives the characteristics of several powders in use, reflecting powders from ten producers [18, 39, 41, 44, 46, 53-67]. 典型的17-4PH不锈钢粉末的中值粒径为10~12μm,振实密度为4.3 g/cm3。表2给出了使用中的几种粉末的特性,反映了来自十个生产商的粉末[18, 39, 41, 44, 46, 53-67]。
Table. 2 Examples of 17-4 PH powder characteristics 17-4PH不锈钢粉末的性能(表中GA就是气体雾化粉、WA就是水雾化粉)
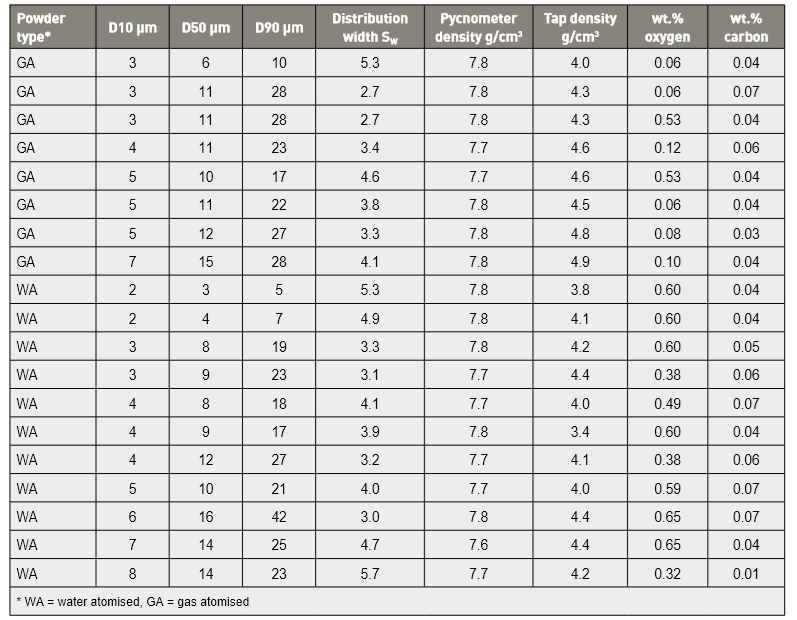
Oxygen is higher in the water atomized powder, ranging up to 0.65%. In contrast, gas atomized powder oxygen content is generally lower, near 0.06%. Some oxygen is removed by reacting with residual carbon during sintering. Residual oxygen degrades corrosion resistance. 水雾化粉末中氧含量较高可达0.65%,相比之下,气体雾化粉末的氧含量一般较低,仅达0.06%。在烧结过程中,可通过与残余碳反应来除去一些氧,过多的残余氧会降低了材料的耐腐蚀性。
The sizes D10, D50 and D90 correspond to the 10%, 50% and 90% points on the cumulative mass-based particle size distribution. Due to doubtful accuracy, particle sizes are rounded to the nearest micrometer sizes. The parameter SW is an indication of the log-normal size distribution (narrow size distributions have a high value corresponding to a steep log-normal slope) [68]. 粉体平均粒径尺寸D10、D50和D90是对应于基于粉末累积品质的细微性分布的10%、50%和90%点。由于简化的准确性,颗粒大小被舍入到最接近的微米尺寸。参数SW是对数正态大小分布的指示(窄的细微性分布具有对应于陡峭的对数正斜率的高值)[68],SW公式如下(公式1):
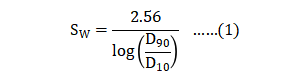
The average SW is similar to that found for many powders. The pycnometer density is the full density for the powder and this is influenced by Ni, Cu, Cr content, as well as C, O, N impurities and any pores inside the particles. The tap density is a precursor to the feedstock solids loading and the green density prior to sintering. 平均SW可以用于许多粉末所发现的,比重计密度为粉末的全密度,这将受Ni、Cu、Cr含量以及C、O、N杂质和颗粒内的任何孔隙的影响。振实密度是原料固体装载和烧结前的生坯密度决定性的前驱物。
Average powder characteristics from about sixty reports are summarized in Table 3. Stainless steel surface chemistry is rich in carbon and oxygen, with upwards of 40 atomic % of each species on the surface [4, 69]. The remaining surface consists of Fe, Si, Cr and Cu, in decreasing order of abundance. For a water atomized powder, the surface consists of Cr2O3, SiO2 and MnO [61]. 在表3中总结了大约60份不同实验报告的平均粉末特性(针对同一支17-4PH不锈钢材料),其表面的化学成分中富含碳和氧,在其表面上至少占有40 atom.%[4, 69],剩余表面由Fe、Si、Cr和Cu组成,含量呈下降趋势。对于水雾化粉末,其表面由Cr2O3, SiO2和MnO[61]等氧化物组成。
Table 3 Typical powder characteristics 17-4HP的典型粉末特性
Master alloy mixtures provide similar properties after sintering to gas atomized powders [70]. For example, hydrogen sintering at 1370°C for 75 min gives a tensile strength of 956 MPa (master) and 954 MPa (gas atomized) with 3.8 and 4.2% elongation. The sintered density is essentially the same at 98%. 母合金混合物在烧结到位是可以逼近气体雾化粉末相似的性能[70]。例如,在1370℃下氢气烧结75分钟,拉伸强度为956 MPa (母合金粉)和954 MPa (气体雾化粉),延伸率为3.8和4.2%。烧结密度在98%时基本相同。
For one binder, water atomized powder with a higher oxygen level reacts with carbon residue from the binder, beneficially reducing sensitivity to de-binding parameters [45]. A comparison of sintered (1300°C, hydrogen) 14 µm gas atomized and 14 µm water atomized powders shows tensile strength of 1280 MPa (pure gas atomized) and 1080 MPa (pure water atomized) with sintered densities of 98.9 and 97.2% [71]. However, this strength difference is eliminated by sintering the water atomized powder at a higher temperature. 对于任一种粘结剂,具有较高氧水准的水雾化粉末与粘结剂中的碳残留物反应,将有利地降低脱脂参数的灵敏度[45](此处指的是含氧量多的粉末可以因此使氧与碳结合而排除粘结剂更干净)。烧结(1300°C,氢气)14μm气体雾化和14μm水雾化粉末的比较显示,拉伸强度为1280 MPa (纯气体雾化)而1080 MPa(纯水雾化),烧结密度为98.9和97.2% [71]。然而,通过在较高的温度下烧结水雾化粉末的试验,便消除了这种强度差异。
Statistical analysis reveals no significant impact of powder characteristics on sintered properties. Mixed gas and water atomized powders are used to lower cost and improve inter-particle friction to resist slumping during de-binding [54, 71, 72]. A higher sintering temperature is required to attain the same density as the proportion of water atomized powder increases. 统计分析表明,粉末特性对烧结后性能没有太显着影响,混合气体雾化和水雾化粉末用于降低成本并改善颗粒间摩擦以防止脱脂过程中的坍塌很有效[54, 71, 72],当水雾化粉末的比例增加后,需要更高的烧结温度以得到没有添加时的相同的密度值。
Sintering parameter effects 烧结参数的影响
Several sintering parameters interact to determine the sintered density, phases, microstructure and properties. Statistical analysis of eighty sintering studies reveals significant impact from four factors - particle size, starting oxygen content, sintering atmosphere and heat treatment. The dominant factor is sintering temperature, accounting for about 90% of the sintered density variation. The powder type, hold time, green density and starting carbon content are not significant factors with respect to sintered density. As detailed in the following sections, experiments designed to test these several factors touch on molding, de-binding, sintering, Hot Isostatic Pressing (HIP) and heat treating parameters. It seems that often the design of experiments is able to isolate effects, but invariably the interaction of parameters and incomplete reporting hide underlying cause-effect relationships. Inherently, in sintering 17-4 PH stainless steel, we must assume everything is important. Here, we treat the adjustable parameters and report their influence on the response parameters of density and properties. 几个烧结参数会相互作用,用以确定烧结密度、相、微观结构和性能。八十个烧结研究的统计分析揭示了四个主要影响的因素:颗粒大小、起始氧含量、烧结气氛和热处理有显着的影响。其中,烧结温度占主导地位,占烧结件密度变化的90%左右;粉末类型、保温时间、生坯密度和起始含碳量对烧结密度则没有显着影响。在下面的部分中详细介绍了实验,这些试验旨在测试这些因素对成型、脱脂、烧结、热等静压(HIP)和热处理参数的影响。似乎这些实验的设计希望能够隔离出一些结果,但是总因参数和不完整报告的交互隐藏潜在的因果关系。因此,在烧结17-4PH不锈钢中,我们必须假定一切都是重要的。在这里,我们处理的可调参数,并报告其影响的密度和性质的回应参数。
Green density生坯密度
In Metal Injection Molding and Additive Manufacturing, the green density is about 96% of the particle tap density. The relation between fractional sintered density fS , green density fG , and sintering shrinkage Y is as follows: 在金属注射成形和增材制造中,生坯密度约是震实密度的96%左右。烧结件的分数密度fS、生坯密度fG和烧结收缩率Y之间的关系如下:
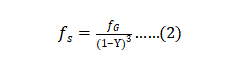
This equation assumes no mass loss during sintering. For stainless steel powder, the usual behavior is that a higher green density produces a higher sintered density [73]. However, contrary to this sense are results using mixed particle sizes to improve green density from 64 to 71% of theoretical. Surprisingly, the lowest green density produces the highest sintered density. This is because of the smaller median particle size for the lower green density. Reports from other studies, including Additive Manufacturing, find that a higher green density does not necessarily result in a significant sintered density gain [74]. 该方程在烧结过程中假设没有质量的损失。对于不锈钢粉末而言,通常的行为是较高的生坯密度就会产生更高的烧结密度[73]。然而,与此相反的是使用混合颗粒大小来提高生坯密度,从理论的64提升到71%,结果令人惊讶的是,最低的生坯密度产却得到了最高的烧结密度。这是因为较低密度的生坯,中值粒径(d50)变小的缘故。来自其他研究的报告,包括增材制造(AM, 3D列印),都发现较高的生坯密度不一定导致显着的烧结密度增益[74]。(不要一味的追求高生坯密度,而是改善粉末粒径的分配)
在过去的工作经验中,以水气雾化的粉末较不规则形状来协助气雾化粉圆球外型的堆叠保形(Conformal)性,并且可以节省气雾化细粉的用量,我们曾经在许多客户的案例上完成外观-结构件产品,便是采用混合粉末得到兼具的特性。
Gas atomized spherical particles enable a higher solids loading and green density. Water atomized powders can approach similar high packing densities, sintering to nearly the same final density. In vacuum sintering (120 min hold at 1390°C), gas atomized powder at 65 vol.% solids loading reaches 98% density, while lower packing density water atomized powder (55 vol.% solids loading) reaches almost the same value at 97% [51]. The latter exhibited more shrinkage. 气体雾化球形颗粒能够提高固体装载量和生坯密度;水雾化粉末也可以接近类似的高堆积密度,烧结到也几乎相同的前者的最终密度。在真空烧结(在1390°C保持120mins)下,气体雾化粉末在65vol%固体负载下达到98%密度,而低堆积密度的水雾化粉末(55vol%固体装载)在97%[51],也达到几乎相同的值。后者表现出更大的收缩。(别忘记密度不是唯一评断产品的重点,收缩大使产品密度变高。但尺寸就会减少)
Another study compares 9 µm water atomized powder with green densities ranging from 55 to 71% [46]. After sintering (60 min at 1365°C), a higher sintered density results from the lower green density. Thus, from a few direct comparison studies, there is little evidence of a dominant green density effect on sintered density, or even sintered properties. Efforts to improve tap density or green density seem to be counterproductive. 另一项研究是采用9μm水雾化粉末与55~71%[46]的生坯密度进行比较。烧结后[1365℃保温60mins],较低的烧结密度反而导致烧结件密度较高。因此,从一些直接的比较研究,很少有证据表明主导的生坯密度对烧结密度的影响,甚至烧结性能的影响。提高振实密度或生坯密度的努力想获得较高的烧结密度似乎适得其反。
高震实密度的粉末一定是越趋于圆形、尺寸细小,因此阻碍了成形和脱脂的顺畅性,结果导致烧结后产品的密度反而不好。在许多粉末厂常常以高震实密度作为判断粉末品质好坏的依据,这是沿袭自传统粉末冶金的作法,但必须要注意到传统粉末冶金的粘结剂很少,也没有辅助压实坯体的填充剂与辅助剂,良好的分配粉末等级以及充分混练得到够好的分散,MIM喂料对于震实密度的要求并不需要极度的高。
Furnace types烧结炉型
A wide variety of sintering furnaces are used to sinter 17-4 PH [75, 76]. The options include laboratory tube, refractory metal batch, graphite batch, and continuous belt, pusher, or walking beam designs. Microwave sintering trials conclude that this is inferior to standard sintering, but the difference is not explained [77]. In a study on molybdenum and graphite furnaces sintering 316L stainless in argon, the final density, yield strength, tensile strength and ductility are essentially identical [76]. The same study compared nitrogen as the atmosphere with a slight density-property gain for the graphite furnace (yield strength 375 MPa versus 330 MPa). However, when vacuum or hydrogen sintering are applied to 17-4 PH, there is no evident furnace role. Sintering is dominated by other factors, especially the peak temperature. Note that some furnace types restrict the atmosphere options, but, to-date, the furnace type is not significant. 各种形式的烧结炉都可用于烧结17-4PH[75, 76]。选择包括实验室管炉、耐火金属批次炉、石墨批次炉、和连续网带炉、推杆炉,或步进梁炉的设计,其中以微波烧结炉试验得出的结论,产品烧成可低于正常标准烧结温度,但差异并没有解释[77](微波炉烧结法的能量很集中,并且有磁场因素的介入,所散失能量很少,不过有许多限制条件,不是小篇幅可以解释的,过去有很多案例都是受到粘结剂的干扰造成微波烧结过程产品炸裂,尺寸忽大忽小,最后没有很明确的结论)。在研究中,钼内胆和石墨内胆炉中烧结通以氩气的316L不锈钢产品时,其最终密度、屈服强度、抗拉强度和延展性基本上两种炉子是相同的[76]。相同的研究比较了氮气作为气氛与石墨炉有着轻微密度特性的增益(屈服强度375 MPa与330 MPa)。然而,当真空或氢气烧结应用于17-4 pH时,没有明显的炉子作用。烧结主要受其它因素的影响,特别是峰值温度。请注意,一些炉类型限制了大气的选择,但到目前为止,炉子类型并不太重要。(哎呀,17-4PH真是乖小孩,完全不挑嘴)
实际在大量化产品烧结时,金属热场的洁净度加上氢气确实优于石墨热场炉,但实验室的烧结炉容积小、放置的烧结件少,差异不会太明显。量产时的真实尺寸效应和数量,对于烧结影响是很大的,必须注意到。
Particle size粉末粒径尺寸大小
Initial particle bonding during sintering is by surface diffusion [78]. Smaller particles have more surface area and, therefore, naturally the initial bonding and strengthening is dependent on particle size. Surface diffusion induced bonding replaces the particle adhesion initially provided by the backbone polymer, so slow heating is a typical protocol to add strength as binder pyrolysis occurs. As the sinter bonds grow between particles, grain boundary diffusion becomes the controlling process. This requires neck growth to form a grain boundary between particles. As grain boundaries form, the rate of sintering shifts to an inverse grain size dependence. Once shrinkage starts, the initial particle size role becomes secondary and attention shifts to the grain size. Even so, particle size is easily monitored as a starting parameter while grain size is less frequently measured. 烧结过程中的初始颗粒结合是通过表面扩散[78]。较小的颗粒具有更大的比表面积,因此,自然的初始结合和强化取决于颗粒大小。表面扩散诱导键合取代最初由主链聚合物提供的颗粒粘附,因此缓慢加热是典型的协议,以维持粘结剂发生热分解时的生坯强度。随着烧结件因颗粒间的成长,晶界扩散成为控制过程。这时需要颈部生长,以形成颗粒之间的晶界。当晶界形成时,烧结速率转变成逆晶粒尺寸依赖性。一旦收缩开始,初始颗粒尺寸的作用就变为次要的,注意力将转移到晶粒尺寸。即使如此,颗粒尺寸很容易被监测作为起始参数,而晶粒尺寸反而是不太频繁被测量的。
In a study with median particle sizes (D50) varied from 5.8 to 12.2 µm, the sintered strength is essentially the same once the peak temperature exceeds 1250°C (60 min, hydrogen) [56, 64]. Sintered densities of 98% or higher are attained for 11 to 12 µm powders when sintered at temperatures over 1288°C. A comparison of 8 and 10 µm particle sizes (vacuum sintered at 1330°C for 60 min) reports a slightly higher density (98.2%) with smaller particles [79]. This promotes a slightly higher yield strength (760 versus 728 MPa) and tensile strength (888 versus 870 MPa) on using the finer powder. Longer hold times or higher peak temperatures tend to offset the early particle size effect. Indeed, for a 60 µm particle size, sintered at 1340°C for 60 min in hydrogen, the density is lower at 92.3%, but the heat treated strength reaches 1100 MPa with a fracture elongation of 2% [80].在一个中值粒径(D50)从5.8~12.2μm变化的研究中,一旦峰值温度超过1250°C(60分钟,氢)[56, 64],烧结强度基本相同。当在1288℃以上的温度下烧结时,11~12μm的粉末达到98%或更高的烧结密度。8~10μm的颗粒尺寸(真空烧结在1330℃时保温60分钟)比较报告密度稍小98.2%,颗粒较小[79]。这促进了使用更细的粉末得到稍微高的屈服强度(760比728 MPa)和拉伸强度(888比870 MPa)。较长的保持时间或更高的峰值温度倾向于抵消早期的颗粒尺寸效应。事实上,对于60μm的粒径,在1340℃下在氢气中烧结60分钟,在92.3%的密度较低,但热处理强度达到1100 MPa,断裂伸长率为2%[80]。(这代表控制的好,粗粉仍旧可以烧到高致密性)
Sintering distortion is lowest with the smaller particle size and largest with the larger particle size; both increase with sintering temperature. This is one justification for smaller particles or even mixtures of different particle sizes or powder types. However, more careful experiments are required to sort out densification versus distortion in cycles optimized for each particle size. To date, the sintering cycle is held constant using differing particle size, but this over-sinters the small particles or under-sinters the large particles. 烧结变形最小,粒径越小;粒径越大,烧结温度越高。这是对于更小的颗粒,甚至是不同粒径或粉末类型的混合物的一个理由。然而,需要更仔细的实验来对每个颗粒尺寸优化的回圈中的致密化与畸变进行排序。到目前为止,以上所有实验的烧结周期是使用不同的颗粒尺寸但保持恒定时间,但不能用于烧结过度小颗粒或烧结过大颗粒。
Heating rate 加热速率
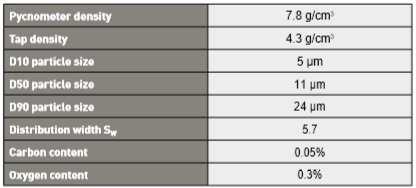
Sintering cycles involve heats and holds to allow binder burnout and impurity removal. Optimal holds are near 600°C for final polymer removal and near 1000°C for impurity reduction. The heating rate approach to the peak temperature ranges from 2 to 10°C/min. When the heating rate drops to 2°C/min, then almost all shrinkage occurs prior to the isothermal hold [81]. As a specific example, for 1270°C, the density on reaching that temperature is 88%, starting from a green density of 55%. Fig. 2 illustrates how the rate of densification declines quickly at the highest temperatures. Most of the densification occurs during heating, especially prior to reaching 1300°C. Indeed, with 2°C/min heating from 1010°C to 1320°C, near full density occurs with a 10 min hold [81, 82]; delivering a tensile strength of 1185 MPa [42]. 烧结回圈涉及加热和保持温度,以允许粘结剂烧毁和杂质去除。最终的聚合物完全去除接近于600°C,杂质完全去除接近1000°C。加热速率接近峰值温度范围为2~10°C/min,当加热速率下降到2°C/min时,几乎所有收缩都发生在等温保持[81]之前。举一个具体的例子,对于1270°C,达到该温度的密度为88%,生坯密度开始是从55vol%的装载量。图2表示出了致密化率如何在最高温度下迅速下降。大多数致密化发生在加热过程中,特别是在达到1300°C之前。实际上,2°C/min的加热从1010°C到1320°C,接近完全密度发生,保持10分钟[81, 82];拉伸强度为1185 MPa[42]。
一样的,提醒大家量产过程要注意到尺寸的效应,譬如脱脂不完成的坯体,对升温效率是很敏感的,过高的升温率可导致坯体开裂、鼓泡甚至发生炸裂,因此不能掉以轻心。厚度越大的产品,心部的位置就越容易残留粘结剂,因此摆放数量、产品形貌,对于升温速率的调整必须经过测试和验证的。
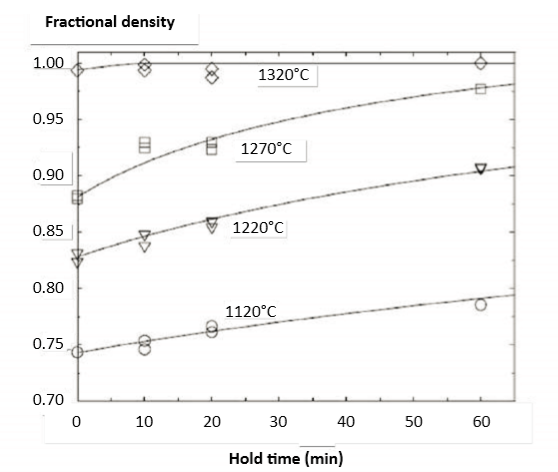
Fig. 2 Data showing sintered density versus hold time after heating to various temperatures [81]. Heating is 2°C/min from 1010°C to the peak temperature. For a peak temperature of 1320°C, almost all densification occurs prior to the isothermal hold. The starting density is 55% using 10 µm gas atomized powder 图上的数据显17-4PH在加热到不同温度后的烧结密度与保温时间的资料[81],加热温度从1010℃到峰值温度为2°C/min升温速率。在1320°C的峰值温度那条曲线,几乎发现致密化已经发生在保温温度之前(也就是只要稍微保温就够了,多的保温时间是浪费),使用10μm气体雾化粉末,起始密度为55vol %。
Temperature 烧结设定温度
When tested using dilatometer, the onset of sintering shrinkage is detected near 900°C [51]. The peak sintering shrinkage rate is near 1250°C [27, 47], but full-density sintering (say 98%) requires peak temperatures approaching 1300°C. However, such high temperatures evaporate copper and chromium, with a potential loss of heat treatment response and corrosion resistance. Delta-ferrite formation is favored by higher temperatures and this phase assists final densification due to faster diffusion. Data on the shrinkage and sintered density (60 min, hydrogen) versus temperature for a 10 µm water atomized powder are plotted in Fig. 3 [59]. Almost the same values were reported in a study with gas atomized powder [53]. At 1350°C, a density of 99% is reached, about the same as achieved with gas atomized powder [47] and, at 1300°C, the density is 96%, which compares favorably with a report of 97% for the same conditions [83]. Shrinkage for 60% green density is 15.4%, similar to another report at 15.7%. Such small differences in dimensional change probably reflect differences in delta-ferrite content. A study starting at 55% green density reports 17.5% shrinkage, giving 98% sintered density. In this case, about 10% delta-ferrite remained. 当使用膨胀计测试时,在900°C[51]附近检测到烧结收缩的开始。峰值烧结收缩率接近1250°C[27, 47],但全密度烧结(98%)需要接近1300°C的峰值温度,但这样的高温会使铜和铬蒸发,从而导致热处理回应和耐腐蚀性的潜在损失。δ-铁素体的形成受较高温度的影响,这一阶段由于更快的扩散有助于最终致密化。在图3(59)中绘制了关于10μm水雾化粉末的收缩和烧结密度(60分钟保温,氢气)与温度的资料。在气体雾化粉末的研究中报导了几乎相同的值[53 ]。在1350°C时,达到99%的密度,与气体雾化粉末[47]相同,在1300°C时,密度为96%,与相同条件下的97%的报告相比有利[83]。60 vol%生坯密度的收缩率为15.4%,类似于另一个报告的15.7%。尺寸变化的这种微小差异可能反映δ-铁素体含量的差异。从55 vol%生坯密度开始的研究报告最后有17.5%收缩,得到了98%烧结密度。在这种情况下,大约10%δ-铁素体仍然存在。
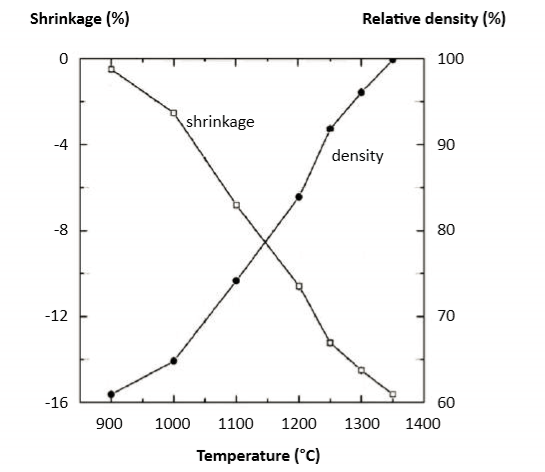
Fig. 3 Plot of sintering shrinkage and sintered density versus hold temperature, heating is at 5°C/min with a 60 min hold at the final temperature [59] 烧结收缩率和烧结密度与保温温度、升温速率为5°C/min,在最终温度下保持60分钟[59]。
The sintering shrinkage curve is a reflection of atomic scale atom motion. Mathematically, the dimensional change is treated as a viscous flow event, similar to how glass deforms at high temperature. For 17-4 PH, the viscosity during sintering is approximated as: 3.4∙107 exp (3305/ T ) Pa∙s, where T is the absolute temperature [84-86]. This is an effective viscosity that includes many factors lumped into a single term. Quenched samples harvested during heating show fully austenitic structures from 780°C up to 1200°C and the emergence of delta-ferrite over 1220°C. Fig. 4 is an example of hydrogen sintered material quenched from 1260°C, showing colonies of delta-ferrite. The pores are transforming to spherical shapes. Property adjustments are possible by mixing austenitic 316L powder with 17-4 PH powder, possibly delivering altered magnetic and mechanical properties [87]. This is an area lacking attention, probably due to the difficulty in qualifying products sintered from mixed stainless steel powders. 烧结收缩曲线是由原子级的运动所反映。在数学上,尺寸变化被视为粘性流动事件,类似于玻璃在高温下变形的方式。对于17-4 PH,烧结过程中的粘度近似为: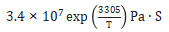 ,其中T是绝对温度[84-86]。这是一种有效的粘度,将许多因素集中在一个公式内。在加热过程中对样品进行的淬火,显示出从780°C~1200°C的完全奥氏体结构以及在1220°C出现的δ-铁素体。图4是从1260℃淬火的氢烧结材料的实例,显示出δ-铁素体的聚落,生坯体的孔隙正在转变为球形。通过将奥氏体316L粉末与17-4 PH粉末混合,可以进行性能调整,可能会改变磁性和机械性能[87]。这是一个缺乏关注的领域,可能是由于难以鉴定由混合不锈钢粉末烧结的产品。(注意非标准的材料必须要求客户签定免责权,否则不要轻易尝试混合)
,其中T是绝对温度[84-86]。这是一种有效的粘度,将许多因素集中在一个公式内。在加热过程中对样品进行的淬火,显示出从780°C~1200°C的完全奥氏体结构以及在1220°C出现的δ-铁素体。图4是从1260℃淬火的氢烧结材料的实例,显示出δ-铁素体的聚落,生坯体的孔隙正在转变为球形。通过将奥氏体316L粉末与17-4 PH粉末混合,可以进行性能调整,可能会改变磁性和机械性能[87]。这是一个缺乏关注的领域,可能是由于难以鉴定由混合不锈钢粉末烧结的产品。(注意非标准的材料必须要求客户签定免责权,否则不要轻易尝试混合)
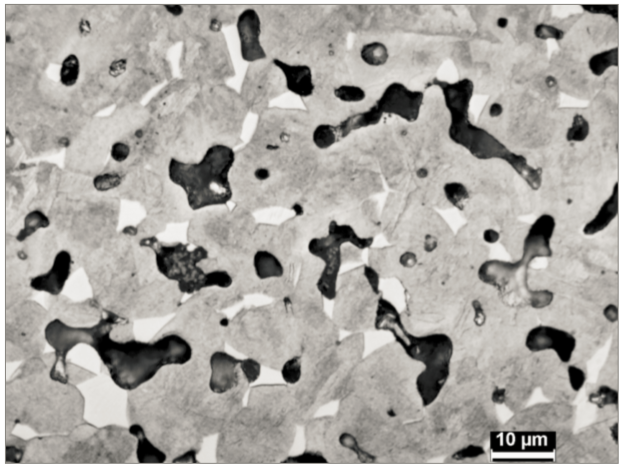
Fig. 4 Microstructure of 17-4 PH water quenched from 1260°C during hydrogen sintering, showing emergence of white delta-ferrite colonies. The dark regions are pores with some small circular oxide inclusions以氢气烧结从1260℃淬火的17-4PH,其微观结构显示出白色δ-铁素体聚落的出现。暗区是具有一些小的圆形且有氧化物夹杂的孔。
Independent trials using 60 min holds in hydrogen are widespread and tend to report similar densities [53]. For example, 1350°C produces 99.4% density for gas atomized 10.7 µm powder. Other studies find equivalent densification at slightly lower temperatures for longer times. Fig. 5 maps interpolated time temperature effects on sintered density for a gas atomized powder [57]. Generally, the sintering studies are in agreement with respect to the time-temperature trade-off. For example, the horizontal one hour trace in this figure shows progressive density gains with increasing sintering temperature. However, long hold times are less productive. A comparison of gas and water atomized powders at 1300°C and 1380°C found both powders sintered to a high density at the lower temperature [71]. Another study comparing 1345, 1360, or 1380°C at times of 60, 90, or 120 min concluded that 1380°C for 90 min resulted in the highest tensile strength (1275 MPa, 36 HRC, 5% elongation) [88]. As previously mentioned, a problem with such a high sintering temperature is evaporative loss of chromium and copper; chromium vapor pressure is 4.5 times higher at 1380°C versus 1300°C and copper vapor pressure is double that of chromium. 使用氢气60分钟保温的独立试验是很普遍的方法,一些报告都反映出类似的密度[53]。例如,1350℃对气体雾化粉粒径是10.7μm粉末烧结后获得了99.4%的密度,其他的研究发现在较低温度下等效致密化时间较长。图5显示了内插时间温度对采用气体雾化粉末体的烧结密度之影响[57]。通常,烧结研究对于时间 - 温度权衡是一致的。例如,该图中的水平一小时迹线显示随着烧结温度的增加而逐渐增加的密度。但是,长时间保持效率较低。将气体雾化和水雾化粉末在1300℃和1380℃下进行比较,发现两种粉末在较低温度下可以烧结成高的密度[71]。另一项在保温60, 90或120分钟时比较1345, 1360或1380°C的研究得出结论,1380°C 90分钟导致最高的拉伸强度(1275 MPa,36 HRC,5%伸长率)[88]。如前所述,这种较高的烧结温度会有问题是铬和铜的蒸发损失;铬蒸气压在1380°C时相对于1300°C高4.5倍,铜蒸气压是铬的两倍。(高温烧结容易对17-4PH表面的铬和铜蒸发,因而导致盐雾测试的快速生锈)
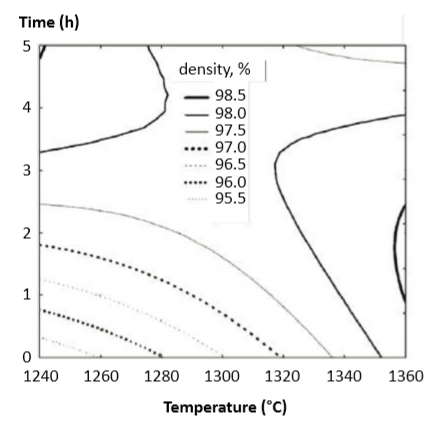
Fig. 5 Map of computer interpolated sintered density contours versus time and temperature [57] 计算机内插法的烧结密度等值线与时间和温度的关系图[57]
Hold times保温时间
Typical sintering hold times at the peak temperature are in the 60 to 120 min range. Little gain comes from the long holds. For one hour, the horizontal line in Fig. 5 indicates a progressive density gain from 96.3% to 98.5% as the temperature increases from 1240 to 1360°C. With about 60 min hold the compacts almost reach full density. As porosity is eliminated, the rate of pore annihilation declines, partly due to exhaustion of porosity and partly due to loss of grain boundaries due to grain growth. Grain boundaries are the annihilation sites for vacancies during sintering. Pores are massive collections of vacancies. Dilatometry shows that the peak shrinkage rate during heating occurs while about 7% porosity remains (near 1250°C) [47]. Longer holds evidence saturation; the densification has gone about as far as possible due to grain size increases and loss of grain boundaries. The results plotted in Figs. 2, 5 and 6 verify that, when 1320-1350°C is reached, the density is high and further gains with extended holds are small. This implies that, upon reaching the ideal sintering temperature range, only a few minutes of hold are required. Most of the densification occurs during heating [51, 81]. 峰值温度下的典型烧结保持时间在60~120分钟范围内,长期保温的收益微乎其微。在一小时之内,如图5中的水平线表示随着温度从1240℃升至1360℃,渐进密度增益从96.3%增加到98.5%。持续约60分钟,坯块就几乎达到全密度。随着孔隙率的消除,孔隙湮没率下降,部分原因是孔隙度被填满耗尽(质量移动,原子的扩散造成质量移动),而部分原因是由于晶粒生长导致的晶界消失。晶界是烧结过程中空位的湮没位置,孔洞是大量物质的空缺。以膨胀计测量表明,加热过程中经过峰值收缩率发生后,孔隙率约为7%(在接近1250°C位置)[47]。证据越来越明显:由于晶粒尺寸的增加和晶界的损失,致密化已尽可能地发生。结果绘制在图2和3中,图2, 5和6证实,当达到1320~1350℃时,密度增高时即使延长保温时间,但产品的密度进一步增益却很小。这意味着,在达到理想的烧结温度范围时,仅需要几分钟的保持时间。大多数17-4PH致密化发生在加热过程中[51,81]。
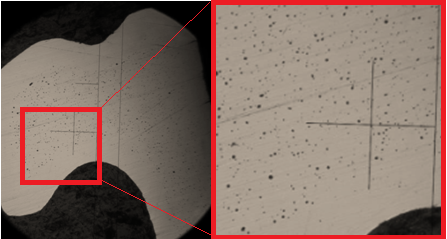
上图是典型的烧结17-4PH显微结构,我们可以发现气孔的分部是沿着边界有一层高密度层包着MIM固有的气孔分部结构,这个孔隙度约为3%具有表层致密的结果,通常要小心抛光和研磨不要超过这个致密层的限度,否则就会发现抛光后出现大量孔洞在抛光面。
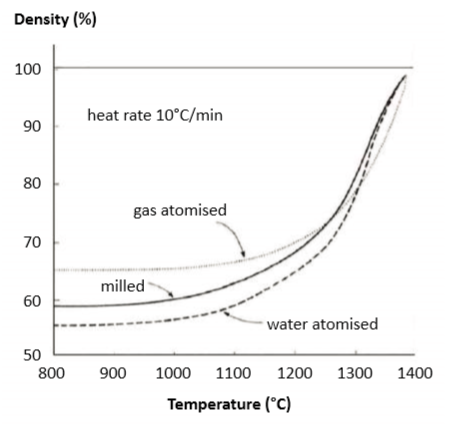
Fig. 6 Constant heating rate dilatometry data for three powder types, heating at 10°C/min [51]. The data illustrate how full density is approached during heating if the peak temperature reaches about 1350°C采用膨胀测量三种粉末类型在恒定加热速率下的热膨胀数据,以10°C / min加热[51]。 数据说明了如果峰值温度达到约1350°C,在加热过程中几乎接近完全密度。
Sintering parameters play a role in compact densification. At first, surface diffusion induces particle bonding. Once necks with grain boundaries form, densification follows by grain boundary diffusion. The model for grain boundary diffusion controlled sintering gives the isothermal shrinkage as a function of the processing conditions as follows [78]: 烧结参数在致密致密化中起了关键作用,首先,表面扩散引起粉末颗粒的结合,一旦形成具有晶界的颈部,便可以迅速通过晶界扩散致密化。晶界扩散控制烧结模型则给出了等温收缩作为加工条件的函数如下[78]:
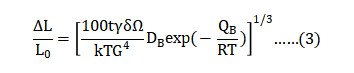
where ΔL is the change in length from the initial size LO , t is the hold time, γ is the solid-vapor surface energy, δ is the atom diameter (assuming the grain boundary is five atoms wide), Ω is the atomic volume, k = Boltzmann’s constant (1.38∙10-23 J/K), T is the absolute temperature, G is the grain size, DB is the frequency factor for grain boundary diffusion, QB is the activation energy and R is the universal gas constant (8.31 J/(mol K)). Such an isothermal model is difficult to test; as illustrated already, since most of the sintering shrinkage occurs before reaching isothermal conditions. Temperature and grain size are dominant parameters in comparison with hold time. ΔL是从初始尺寸L0开始的长度变化值、t是保温时间、γ是固体蒸汽表面能、δ是原子直径(假设晶界宽度为5个原)、Ω是原子体积、k =玻尔兹曼常数(1.38∙10-23 J / K)、T是绝对温度、G是晶粒尺寸、DB是晶界扩散的频率因子、QB是活化能、R是通用气体常数(8.31 J /(mol K))。 这种等温模型很难测试;如已经说明,大部分烧结收缩在达到等温条件之前发生。 与保持时间相比,温度和晶粒尺寸是主要参数。
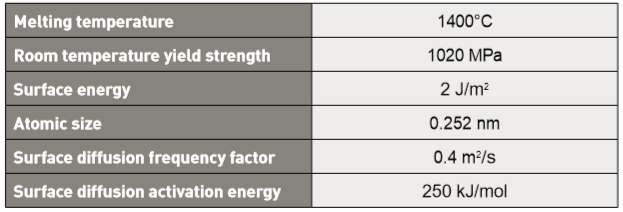
Trials to extract an apparent activation energy from shrinkage data result in values ranging from 312 to 350 kJ/mol [54, 81]. This is slightly higher than 167 kJ/mol reported for other stainless steels [78]. For 17-4 PH, the simultaneous surface diffusion acts to exhaust surface energy, but does not contribute to densification. The result is a high apparent activation energy; surface diffusion acts to form interparticle necks containing grain boundaries, but grain boundary diffusion is only monitored by shrinkage. Further, grain growth goes hand-in-hand with sintering densification, since mass transport across the grain boundary produces grain growth while mass transport along the grain boundary produces densification. Finally, the activation energy extracted from densification data is complicated by phase changes during heating [89]. Surface diffusion provides compact shape retention by building bonds between particles as the backbone binder evaporates. The resulting open pore structure is captured in Fig. 7. Neck growth by surface diffusion under isothermal conditions is modelled as follows [78]: 从收缩资料中提取表面活化能的试验结果为312~350 kJ / mol [54, 81],这比起其他不锈钢报导的略高于167 kJ / mol [78]。对于17-4 PH而言,表面扩散的同时和表面能的作用并不会导致致密化,但是高表面活化能与表面扩散作用形成含有晶界的颈部生成才是致密化的关键,而晶界扩散则仅能通过收缩来监测。不过,晶粒生长与烧结致密化则有密切关系,因为穿过晶界的品质传递造成晶粒生长,而沿晶界的品质传递产生致密化。最后,从致密化资料中提取的活化能因加热过程中的相变而变得复杂[89]。当骨架粘合剂蒸发时,表面扩散通过在颗粒之间建立粘合来提供紧凑的形状保持。得到的开孔结构如图7所示,在等温条件下通过表面扩散的颈部生长模拟如下[78]:
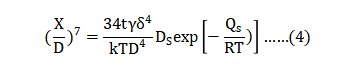
The neck diameter is X , particle diameter is D and X/D is the neck size ratio. In Fig. 7, the neck size ratio varies from 0.3 to 0.5, averaging 0.38. In this equation, t = isothermal hold time, γ = surface energy, δ= atomic spacing, k = Boltzmann’s constant (1.38∙10-23 J/K), T is the absolute temperature in kelvin, DS is the surface diffusion frequency factor, QS = the activation energy for surface diffusion and R = universal gas constant (8.31 J/(mol K)). Data for 316L stainless steel provide a means to estimate surface diffusion neck growth since 17-4 PH is also austenitic during heating. In turn, neck growth by surface diffusion provides a means of estimating strength evolution during heating. Table 4 summarizes the calculation parameters. 颈部直径为X、粒径为D,则X / D为颈部与粉末粒径尺寸比。 在图7中,颈部尺寸比从0.3到0.5变化,平均为0.38。 在这个等式中,t =等温保持时间,γ=表面能,δ=原子间距,k =玻尔兹曼常数(1.38∙10-23 J / K),T是以凯氏温度表示的绝对温度,DS是表面扩散频率因子 ,QS =表面扩散的活化能,R =通用气体常数(8.31 J /(mol K))。 采用316L不锈钢的数据提供了一种估算表面扩散颈部生长的方法,因为17-4 PH在加热过程中与316l相同也是奥氏体。反过来,通过表面扩散的颈部生长提供了估计加热期间强度演变的手段。 表4总结了计算参数。
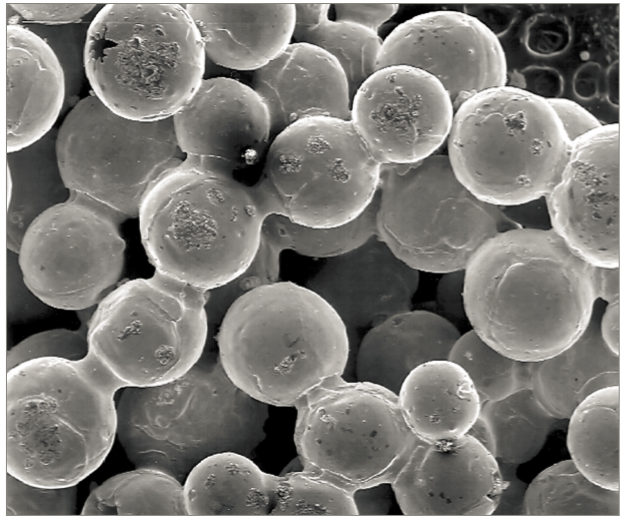
Fig. 7 Spherical particles form necks by surface diffusion early in sintering to provide strength to the compact without contributing to densification在烧结的早期阶段,球状的颗粒粉末颈部形成来自于表面的原子扩散,这样使得产品升坯在没有致密化发生但仍具有强度
Table 4 Surface diffusion controlled strength evolution calculation parameters表面扩散控制强度演变计算参数
Compact strength evolution involves both neck growth and densification. During densification, the number of particle-particle contacts increases, measured by the coordination number [90]. Initially the bond size determines strength [91], but densification induces new bonds and increases the load bearing cross-sectional area. Thus, sintered strength σ is predicted as follows: 生坯强度的演变涉及颈部生长和致密化。在致密化过程中,粉末粒子 - 粒子之间接触的数量逐渐增加,可通过配位数[90]测量得到。最初,低温时粘合剂尺寸决定了强度[91],但致密化会引起新的粘合并增加承载横截面积。因此,预测烧结强度σ如下:
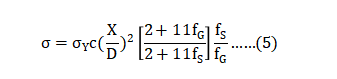
As density increases, due to grain boundary diffusion, the sintered fractional density fS increases from the green fractional density fG . The yield strength for dense material σY is 1040 MPa, a typical value for dense 17-4 PH. The neck size ratio X/D is limited to a maximum of 0.51. At this point, neighboring necks converge and pores are eliminated. The parameter C adjusts to the particular means for measuring strength; it is 1.0 for tensile and 1.6 for transverse tests. 随着密逐渐度增加,由于晶界扩散,烧结坯分数密度fS会增加变成生坯分数密度fG。 致密材料的屈服强度σY为1040MPa,是致密的17-4PH典型值。颈部尺寸比X / D限制为最大0.51。此时,相邻的颈部会聚并且毛孔被消除。参数C调整到测量强度的特定装置; 拉伸为1.0,横向试验为1.6。
Combining equations leads to the plot of expected sintered strength (at room temperature) versus sintering temperature in Fig. 8. The symbols are experimental results taken from different 60 min sintering holds for 10 µm powders [44, 56, 92-94]. Since heat treatment adds complexity, only as-sintered data are included. Grain size data are generally not reported, but this is another factor, as is the formation of delta-ferrite. 组合方程导出后如图8中预期的烧结强度(在室温下)与烧结温度的关系图,方形红点符号是取自不同实验的10μm粉末保温60分钟烧结的实验结果[44, 56, 92-94]。 由于热处理增加了复杂性,因此本图仅使用只有烧结后的数据做为统计,也不报告晶粒尺寸数据,同样的避免复杂,δ-铁素体的形成也是在此省略。
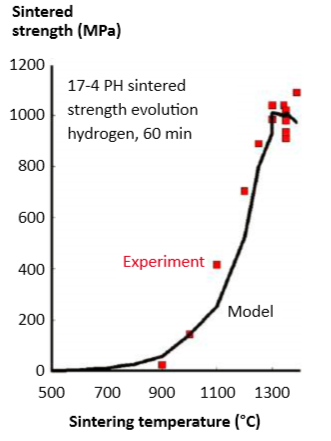
Fig. 8 Comparison of measured tensile strength (square symbols) and surface diffusion predicted strength (model) evolution for 60 min sintering in hydrogen at various temperatures. Experimental data are scattered due to factors such as delta-ferrite content and grain size effects 测量拉伸强度(方形红点符号)和表面扩散预测强度(模型)演变的比较,在不同温度下在氢气中烧结60分钟。由于δ-铁素体含量和晶粒尺寸效应的因素,实验数据落点是分散的。
Note that these calculations provide a means for sensing the desired minimum temperature required during thermal de-binding. Setting a goal of 20 MPa handling strength, the model finds that a peak temperature of 600°C is sufficient, a temperature often encountered in thermal de-binding, as noted earlier. 注意,这些计算提供了一种用于检测热脱脂所需至少最低温度的方法。设定20MPa处理强度的目标,模型发现600℃的峰值温度就足够了,这是热脱脂经常遇到的温度,如前所述。
Atmosphere气氛
The sintering atmosphere options include hydrogen, nitrogen, hydrogen-nitrogen, argon-hydrogen and vacuum. Nitrogen is a potent austenite stabilizer, so it plays a role in martensite formation and final properties [44-46]. The same atmospheres are also used in second stage de-binding. 烧结气氛选项包括氢,氮,氢-氮,氩-氢和真空。氮是一种有效的奥氏体稳定剂,因此它在马氏体形成和最终性能中发挥作用[44-46]。在第二阶段脱脂中(600℃以下的减压脱脂)也使用相同的气氛。
Hydrogen or vacuum are common sintering atmospheres for 17-4 PH stainless steel [95]. Without added graphite, both chromium and silicon retain oxygen. If carbon is added, significant oxide reduction is possible. With respect to atmospheres, a -40°C dew point is possible. This corresponds to an inlet atmosphere with one part moisture for 10,000 parts hydrogen [75]. As oxides are reduced, the resulting reaction product increases dew point, halting further reduction. To compensate, the moisture must be flushed to continually replenish with fresh atmosphere. Thus, both atmosphere quality and atmosphere flow rate are factors. Often, neither parameter is reported. 对于17-4 PH不锈钢,氢气或真空是常见的烧结气氛[95]。粉末中如没有添加石墨,则铬和硅两元素都能保留氧气;如果添加碳,则可以显着减少氧化物。对于保护气压的气氛,需要达到-40°C的露点。这相当于入口大气,一份水分可以变成为10,000份氢[75]。随着氧化物的还原,所得反应产物增加了露点,并停止了进一步的还原。为了补偿这现象,必须冲洗湿气以持续补充新鲜的气氛。因此,大气质量和大气流速都是因素。不过在这些实验中,两个参数都没有特别报告。(在大中华境内由于采用批次真空石墨烧结炉,几乎没有谈到露点,这真的要拜炉膛采用全石墨所致,尤其目前业界习惯有一段600~1000℃的真空内烧,大大的改善了粉末氧含量,石墨气氛都把氧抓走了~)
The dew point is a measure of the temperature at which an atmosphere is chilled to produce condensation. A low dew point is required to reduce surface oxides. The relation between dew point and volume percent moisture is summarized in Table 5. As plotted in Fig. 9, oxide reduction is favored by higher temperatures and higher hydrogen contents [96]. A low dew point ensures oxide reduction. After sintering, the cold surfaces oxidize to form a passive layer that is rich in iron, chromium and silicon [4]. This passive layer provides corrosion resistance. 露点是冷却大气以产生冷凝水的温度度量值,降低表面氧化物需要降低露点,露点和体积百分比水分之间的关系总结在表5中。如图9所示,更高的温度和更高的氢含量有利于氧化物的还原[96],低露点可确保氧化还原。烧结后,冷表面氧化形成富含铁,铬和硅的钝化层[4],该钝化层提供耐腐蚀性。
Table 5 Relation between dew point and atmosphere moisture content露点与大气含水量之间的关系
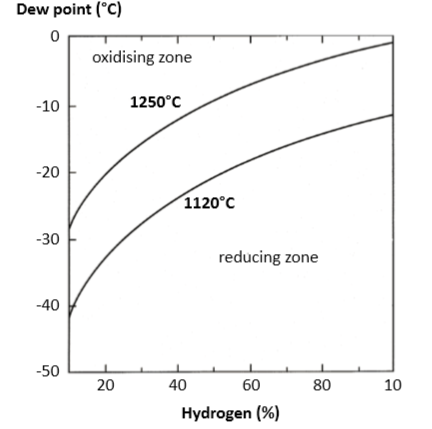
Fig. 9 Plot of the oxidation-reduction boundary for chromium in a stainless steel versus atmosphere hydrogen-nitrogen composition for 1120 and 1250°C [96]. A high hydrogen content ensures reduction in an atmosphere containing higher levels of water vapor不锈钢中铬的氧化还原边界对比1120和1250°C的在保护气氛为氢-氮组成[96]。氢含量高可确保在含有较高水蒸气的气氛中金属得以还原。
Sintering in hydrogen is sensible for carbon and oxygen removal, but vacuum sintering is quite successful. Vacuum sintering causes evaporation of chromium and copper at high temperatures that is suppressed by an atmosphere. Sometimes the atmosphere pressure is below 0.1 MPa, using variants such as argon or hydrogen-argon. However, the sintered density is lowered by argon since it is insoluble and remains trapped in closed pores; a peak density of 95% is typical [95]. A comparison of hydrogen, vacuum and argon sintering (1350°C for 60 min) confirms argon as being inferior (94.6% dense) [97]. 氢气烧结对碳和氧的去除是很敏感的,但真空烧结非常容易成功。真空烧结容易造成铬和铜在高温下蒸发,可以用气氛的增加来抑制,我们会使用诸如氩气或氢气-氩气的混合气体,压力低于0.1MPa~100kPa。 但是,烧结密度会因氩气而降低,因为它不溶于金属中,并且仍然被困在封闭的孔中。采用氩气烧结的典型峰值密度为95%[95],氢气、真空和氩气烧结(1350℃,保温60分钟)的比较证实仅有使用氩气较为差(94.6%致密)[97]。
Nitrogen is soluble in austenite and, on cooling, forms Cr2N precipitates. Since the precipitate robs grain boundary areas of chromium, localized rapid corrosion occurs. Indeed, rust is found within days after sintering in nitrogen. This difficulty is minimized by avoiding nitrogen in the sintering atmosphere or by cooling at 200°C/min below 900°C to suppress chromium nitride formation [98]. 氮可溶于奥氏体,并且在冷却时形成Cr2N沉淀物。 由于沉淀物剥离了铬在接近的晶界区域,因此发生局部快速腐蚀(容易形成晶间腐蚀)。实际上,在氮气中烧结后几天内可以发现烧结的产品生锈。 通过避免烧结气氛中的氮或通过在900℃时以200℃/ min冷却快速降温来抑制氮化铬的形成,可以最大限度地减少这种问题[98]。(所以急冷对17-4PH通过盐雾测试是最有效的热处理法)
Trials with nitrogen additions to the sintering atmosphere fail to demonstrate any notable advantage [44, 46, 70]. Nitrogen is soluble in austenite at temperatures over about 1000°C. The equilibrium solubility depends on temperature and nitrogen partial pressure. Sintering a fine water atomized powder in an atmosphere of 96% hydrogen and 4% nitrogen at 1350°C results in about 0.1 wt.% nitrogen in solution. The final nitrogen content depends on the cooling rate near 900 to 1000°C, the region of maximum solubility. Adsorbed nitrogen has a small negative impact. For example, the sintered density is 97.7 with 96% H2 - 4% N2 and 98.3% with pure H2 and 99.0% with vacuum [44]. In a test matrix of different powder types and sintering atmospheres the findings were mixed [70]. The highest yield strength and corrosion resistance came from master alloy powder sintered in hydrogen (1370°C, 75 min), but the highest ductility and tensile strength came from gas atomized powder sintered in nitrogen. 添加氮气到烧结气氛的试验未能显示出任何显着的优势[44,46,70]。在超过约1000℃的温度下,氮可溶于奥氏体中,其平衡溶解度取决于温度和氮分压。在96%氢气和4%氮气氛中在1350℃下烧结细水雾化粉末导致溶液中约0.1重量%的氮。最终的氮含量取决于接近900~1000℃的冷却速率,即最大溶解度的区域。吸附氮对负面影响很小,例如,烧结密度为97.7 (96%H2-4%N2)和98.3%(纯H2),99.0%真空[44],在不同粉末类型和烧结气氛的测试基质中,研究结果是混合的[70],其中最高屈服强度和耐腐蚀性来自在氢气中烧结的中间合金粉末(1370℃,75分钟),但最高的延展性和拉伸强度来自在氮气中烧结的气体雾化粉末。(在2017年流行于大陆华南的无磁性17-4PH是以全程氮气的方式在真空炉中烧结得到的调值材料,可以获得全无磁性的奥氏体,减少δ-铁素体且在碳少时就不会得到马氏体,不过机械性质低于316L,一但热处理到高温又回到硬度高时磁性又回来,但防锈的能力整体比较原来的高硬度时稳定)
仍旧要提醒读者,高氮无磁性的17-4PH没有被规范的记载,属于不安定相的不锈钢,除非获得客户签订的免责权声明,否则别轻易出货给客户以免将来发生争议。
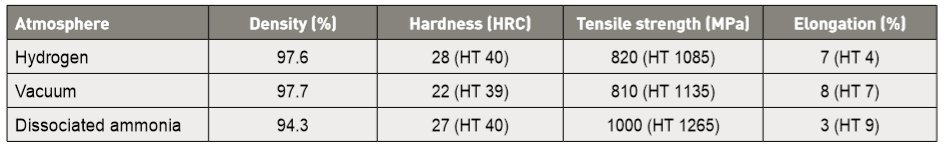
On the other hand, comparison of vacuum, hydrogen and dissociated ammonia sintering (10 µm water atomized powder) results in a lower sinter density (1320°C, 120 min) from dissociated ammonia [94]. Summary properties are given in Table 6 for sintered and heat treated conditions (parenthetical values). A higher temperature, 1370°C for 75 min, gives the opposite result for gas atomized powder [70]. Sorting out such confusion between studies requires more of the details on residual oxygen, carbon, delta-ferrite and grain size. With dissociated ammonia, increasing the sintering temperature to 1350°C results in a high hardness and strength after heat treatment (HRC 44, 1350 MPa), but elongation remains low at 3%. Since nitrogen stabilizes austenite, which produces martensite on cooling, it should be beneficial to heat treated properties. 另一方面,真空,氢和裂解氨烧结(采10μm水雾化粉末)的比较,发现使用裂解氨的烧结件获得较低烧结密度(1320℃,保温120min)[94]。表6给出了烧结和热处理条件(括号值)的总结性质,更高的温度,1370°C,保温75分钟,对气体雾化粉末产生相反的结果[70]。排除研究之间的混淆需要,必须关心更多关于残余氧、碳、δ-铁素体和晶粒尺寸的细节。对于裂解氨而言(含有较多氮气),烧结温度必须提高到1350℃才能在热处理后获得高硬度和强度(HRC44,1350MPa),但伸长率则会保持低至3%。由于氮气能稳定奥氏体(减少δ-铁素体),使含碳高的奥氏体在冷却时产生马氏体,因此对热处理性能应该是有益的。
Table 6 Comparative sintering atmosphere (1320°C, 120 min) effect on 10 µm water atomized 17-4 PH stainless steel as-sintered and heat treated (in parentheses) 表6.对烧结和热处理的10μm水雾化17-4 PH不锈钢的比较烧结气氛(1320°C,120 min)(括号内)。其中 Disociated Ammonia即为裂解氨,可以得到75%氢气和25%氮气 = NH3)
Graphite vacuum furnaces are quite successful in vacuum sintering. Graphite enables the creation of a low partial pressure of carbon monoxide inside the vacuum. Chromium oxide is reduced by CO when the partial pressure is a few hundred parts per million. For example, at 1300°C, a low CO partial pressure (0.001 atmospheres) is reducing to chromium. 在石墨真空炉进行真空烧结非常成功,石墨能够在真空烧结时产生低的一氧化碳分压。 当分压为百万分之几时,氧化铬被CO还原。例如,在1300℃时,低CO分压(0.001个大气压)使氧化铬还原成铬。
Additives添加剂
Several additives allow manipulation of sintering. For 17-4 PH stainless steel, the additives include molybdenum, graphite, boron, silicon, iron boride and nickel boride [59, 60, 94, 99-105]. Molybdenum additions provide some hardening and strengthening when sintering is in dissociated ammonia. Silicon (1%) and boron (0.2%) combine to deliver a heat treated tensile strength of 1200 MPa, but low ductility. Boron segregates to grain boundaries where it forms a liquid phase to lower the sintering temperature. For a gas atomized powder, 0.5 wt.% to 0.6 wt.% boron lowers the sintering temperature to 1250 to 1260°C. After heat treatment, boron doped material reaches a hardness of 55 HRC and 1520 MPa tensile strength. Alternatives are nickel or iron borides; 1 wt.% FeB or 1 wt.% NiB deliver full density using vacuum sintering at 1285°C for 45 min. These compositions heat treat to 1300 to 1400 MPa tensile strength and 50 to 52 HRC hardness, with about 7% fracture elongation. Of concern is that the liquid phase induced by boron probably gives more distortion during sintering. 几种添加剂可以控制烧结,对于17-4 PH不锈钢,添加剂包括钼、石墨、硼、硅(硅),硼化铁和硼化镍[59, 60, 94, 99-105]。当在裂解氨中烧结时,钼添加物可提供一些硬化和强化。硅或称硅(1%)和硼(0.2%)能促进相的结合,可提供1200 MPa的热处理拉伸强度,但延展性则低,硼易偏析到晶界,在晶界处形成液相以降低烧结温度。对于气体雾化粉末,0.5~0.6wt%的硼将烧结温度降低至1250~1260℃,在热处理之后,硼掺杂材料达到55HRC的硬度和1520MPa的拉伸强度。替代品是镍或硼化铁;使用在1285℃下真空烧结45分钟,1wt%FeB或1wt%NiB提供全致密化可能。这些组合物热处理至1300至1400MPa的拉伸强度和50~52HRC的硬度,具有约7%的断裂伸长率。值得关注的是硼引起的液相可能在烧结过程中产生更多的变形。
Graphite additions remove oxygen from water atomized powders. Starting with 0.32% oxygen (and 0.048% carbon), the addition of 0.26 wt.% graphite results in a final composition of 0.01% O and 0.03% C. Fig. 10 is a plot of the final carbon and oxygen levels as influenced by the added graphite content [61]. Notable are the high mechanical properties that result, giving 40 HRC, 1300 MPa tensile strength and 9% elongation after heat treatment. Some corrosion resistance is lost if the final carbon level exceeds 0.07%. 石墨添加剂从水雾化粉末中除去氧气,从0.32%氧气(和0.048%碳)开始,添加0.26重量%的石墨导致最终组成为0.01%O和0.03%C。图10是受最终碳和氧气水平影响的图表。添加的石墨含量[61]。值得注意的是产生不错的机械性能,在热处理后提供40 HRC,1300 MPa拉伸强度和9%伸长率。但是,最终碳含量超过0.07%时,则会损失一些耐腐蚀性。
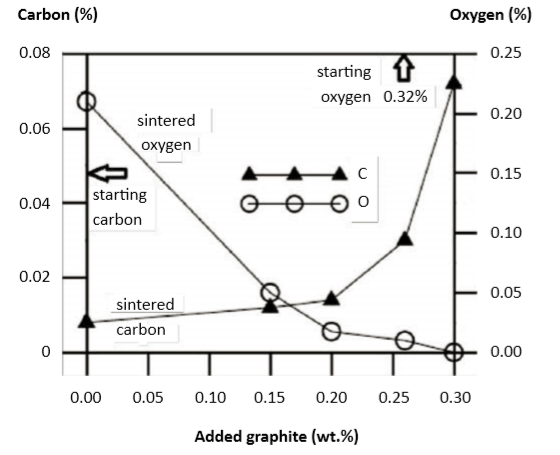
Fig. 10 Influence of added graphite on the sintered carbon and oxygen contents for water atomized 15 µm 17-4 PH stainless steel sintered in graphite vacuum furnace at 1320°C for 60 min [61] 添加石墨对烧结于1320°C石墨真空炉中的水雾化15μm之17-4PH不锈钢烧结的碳和氧含量影响[61] (添加石墨的量有助于改善产品的氧含量,因为生成一氧化碳而抢走金属氧化物中的氧,对于铬和铜的还原有很大的帮助)
Distortion 扭曲
Conceptually, the sintering shrinkage is uniform if the particle packing in the green body is uniform. However, anisotropic shrinkage arises from several factors, including substrate friction along the shrinking bottom surface, gravity induced slumping or bending of unsupported regions, powder-binder separation in molding and non-uniform heating. It is best to use a high green density to minimize distortion [65, 66]. Careful inspection shows sintering shrinkage is not uniform; variations of 0.9% are reported [106]. 具体而言, 如果生坯中的颗粒是均匀的分布, 烧结收缩就是均匀的。然而, 各向异性的收缩是由几个因素造成的, 包括生坯接触底面的衬底与陶瓷板的摩擦、升坯上没有支撑区域的重力引起的滑塌或弯曲、成型中的粉末粘结剂分离和不均匀加热。最好使用高密度生坯来获得最小化的扭曲[65, 66]。仔细检查表明烧结收缩不均匀性,大约是0.9%的变动报告[106]。
An issue during sintering is thermal softening. Sinter bonds between particles add strength, but high temperatures soften the material. Stainless steels decrease strength to reach zero measurable strength near 1400°C. For 17-4 PH, the full density yield strength (in MPa) at temperature σT depends on the absolute temperature T (in K) as follows: 烧结过程中的一个问题是热软化。烧结件在颗粒间增加强度, 但到高温时材料致密后却开始软化,通常不锈钢在1400°C 附近会降低强度, 可测量强度甚至达到零。对于17-4 PH值, 全密度屈服强度(MPa)在温度σT取决于绝对温度T(K凯氏温标) 如下:
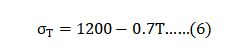
Heating induces bonding followed by densification. Both effects strengthen the powder compact, but heating causes thermal softening of the alloy. This latter effect gives inherent strength loss and less distortion resistance. Substitution of σT for σY in Equation 5 illustrates the susceptibility to distortion and damage during heating. 加热诱导粘结脱除后再致密化,两种效应都为了增加了粉末形成致密体, 但继续加热却也导致合金的热软化,软化作用导致固有的强度损失和变形的抵抗变小。σT 在方程式(5)中的替代σY 说明了加热过程中畸变和强度损失。
Fig. 11 plots the in-situ strength versus temperature during sintering. Contrast these data with the earlier plot showing the room temperature strength after heating to those temperatures. Below about 1200°C, sinter bonding and densification improve strength at temperature, but, over 1200°C, thermal softening dominates, resulting in a net strength loss at temperature during densification. These calculations build from hot rupture data acquired during sintering using a model that evokes surface diffusion neck growth, thermal softening and component densification [107-109]. 图11对烧结过程中的原位强度(意思就是记录在某一温度时的强度值)与温度进行了对比。对比这些资料与早先图显示室温强度在加热到达观察温度,下面关于 1200°C, 烧结粘结和致密提高温度的强度, 但超过 1200°C后, 热软化成为主导坯体净强度损失的关键,即使到达致密化温度强度仍旧掉下。这些计算建立在烧结过程中停等某一温度使坯体破裂所记录下来的数据, 这些阶段包含表面原子扩散、颈部生长、热软化和坯体致密化[107~109]。(大家一定会半信半疑,最高的强度为何不是在致密化阶段?注意到本实验称为原位强度,以当时某温度坯体强度做比对,注意到烧结件密度没有致密时,产品强度虽高但因为孔隙多和尺寸不符合,工程上的应用是不考虑的)
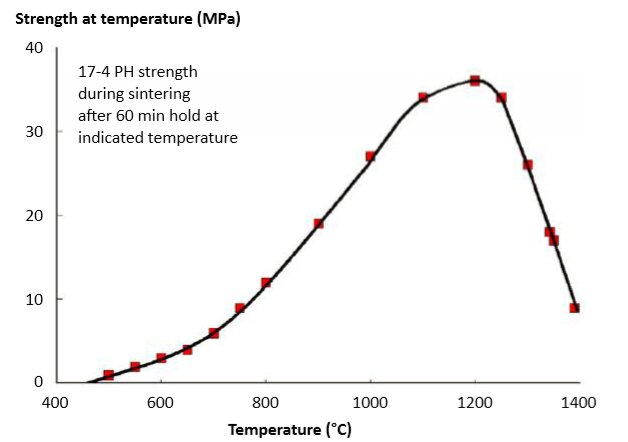
Fig. 11 Strength evolution at various temperatures during sintering, showing the in situ strength after 60 min hold. The greatest resistance to distortion is near 1200°C. Strength loss at higher temperatures is due to thermal softening. Below 600°C the compacts are delicate since the binder has evaporated but surface diffusion has not induced significant particle bonding 在不同温度下烧结并保温60分钟后时的强度演化,显示出原位强度,最大的抗变形能力在接近1200°C,随后在更高的温度引起热软化造成强度的损失。在600°C 以下的生坯体表现很有趣, 因为粘结剂逐渐的蒸发, 但表面扩散并没有引起显着的颗粒粘接强度的增加(仅缓慢增加)。
Based on experiments to extract the sintering response, using variations in time, temperature and heating rate, viscous flow models allow prediction of final size and shape [81, 82, 86]. The models employ finite element analysis. The calculations consistently show densification occurs prior to slumping. Example results are plotted in Fig. 12 for right circular cylinders in profile [81]. The geometry is initially 10 mm in diameter and 10 mm high. Gravity is acting in the vertical direction to cause compact distortion near the bottom. Initially shrinkage is uniform, but, late in sintering, distortion occurs. The shrunk bottom surface is pinned by the compact mass, leading to bowing into the “elephant foot” geometry predicted here. Thus, distortion is reduced by shorter hold times at the peak temperature. Further study is needed to optimize the densification and distortion maps versus peak temperature, hold time and heating rate. 通过实验累计出烧结反应, 对应于时间、温度和加热速率的变化,粘性流模型可以预测最终尺寸和形状 [81, 82, 86]。模型采用有限元分析,计算结果显示了升坯致密化发生在强度暴跌之前,结果在图12中绘制为右圆柱形的轮廓 [81]。几何形状最初是10mm直径和10mm高,重力作用于垂直方向, 导致近底部的致密化后畸变。最初的收缩是均匀的, 但是, 在烧结后期, 变形发生,收缩的底部表面由生坯体的底部被固定, 导致屈服发生于这里,预测出的 "大象脚" 几何形状。因此, 在到达峰值温度下, 变形减少必须要使保温时间缩短。需要进一步的研究是关于如何优化生坯致密和失真的交互作用图与峰值温度, 保温时间和加热率之间的关系。
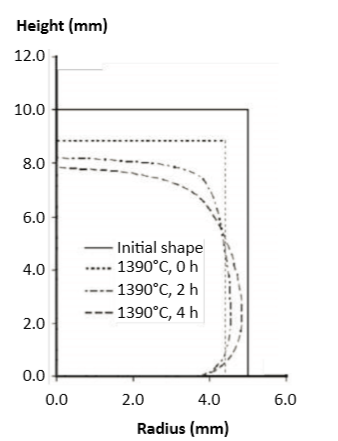
Fig. 12 Finite element analysis predictions on shape distortion for 10 mm right circular cylinders during sintering at 1390°C [81]. The left half of the cylinder is shown for the initial shape at the start and after hold times of 0, 2, and 4 hours 以1390°C [81] 烧结 10 mm 的右圆柱形状,有限元分析预测气缸的左半部分显示畸变的情形,依序为为烧结前的初始形状和开始烧结后温度到达保温时间由0, 2, 4 小时的形状变化。
Carbon control碳控制
Carbon is an austenite stabilizer during sintering. Fig. 13 plots this effect showing the delta-ferrite formation versus carbon content for 17-4 PH [110]. A low retained carbon level, especially below 0.1 wt.%, ensures a higher strength and hardness [97]. Changes in carbon level impact phases, densification and mechanical properties. 在烧结过程中, 碳是奥氏体稳定剂。图13绘制了这一效应,显示17-4PH不锈钢的δ-铁素体的形成与碳含量[110]。低含碳量水准,特别是低于 0.1wt %, 可以确保材料具有更高的强度和硬度[97]。含碳量影响相的变化、致密化和力学性能。
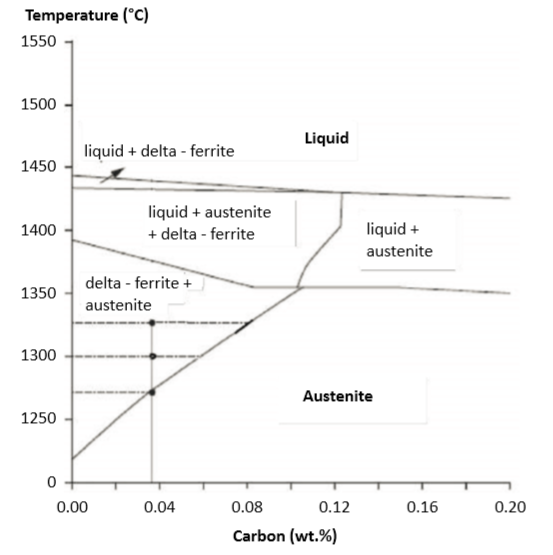
Fig. 13 Relative phase content at various sintering temperatures is indicated by the tie lines on the phase diagram for 17-4 PH stainless steel [110]. Illustrated here are the tie lines at a carbon content of 0.036%. At this concentration, first delta-ferrite forms near 1270°C. By 1325°C the structure is about half austenite and half delta-ferrite. The relative content is verified by quenching during sintering在不同烧结温度下的相对相碳含量由 17-4 PH 不锈钢的相图上的领带线表示[110]。这里说明的是领带线在0.036% 的碳含量。在这个浓度, 第一个δ-铁素体形成附近的1270°C;由1325°C 结构是大约半奥氏体和半δ-铁素体。烧结过程中采用淬火保留高温相的相对碳含量来做验证。
The carbon level depends on the starting powder, binder residuals, de-binding cycle, sintering atmosphere, initial oxygen and peak sintering temperature. A large factor is the initial oxygen level, since carbon and oxygen react during sintering [17, 95]. For example, 0.03% carbon loss occurs in gas atomized powder sintered at 1343°C (60 min) in hydrogen [55]. On the other hand, carbon contamination arises from some binders, impacting densification, especially at lower sintering temperatures. Fig. 14 plots an example of sintered density variation with carbon level. These data are for 11 µm gas atomized powder sintered at various temperatures for 100 min, where the carbon level is adjusted by the de-binding conditions [47]. Further insight is offered in Fig. 15, a plot of heat treated tensile strength versus retained carbon for a variety of hydrogen and vacuum sintered compacts [97]. The tensile strength is for both the sintered (1300 to 1350°C for 60 min) and H900 heat treatment. As the carbon concentration reaches higher levels, a notable loss of strength is evident. 含碳量取决于起始粉末、粘结剂残留、脱粘过程、烧结气氛、初始氧含量和峰值烧结温度,其中最大一个因素是粉末最初的氧含量,因为碳和氧气在烧结期间发生反应[17, 95]。例如, 0.03% 碳损失发生在气体雾化粉末烧结在1343°C (保温60 分钟) 在氢气氛下[55];另一方面, 碳污染来自于于一些粘合剂残留, 影响了致密化的进行, 特别是在较低的烧结温度。图14绘制了含碳量与烧结密度变化的实例。这些资料是采用11µm 气体雾化粉末,在不同的烧结温度分别保温100分钟, 其中含碳量的调整来自脱脂[47]。在图15中提供了进一步的洞察力, 这是一个热处理的拉伸强度与碳含量的关系,生坯于各种氢气和真空烧结条件下[97],拉伸强度为烧结(1300~1350°C,保温60分钟)并采用H900热处理后。当烧结件碳含量较高时, 强度的损失是非常明显的。
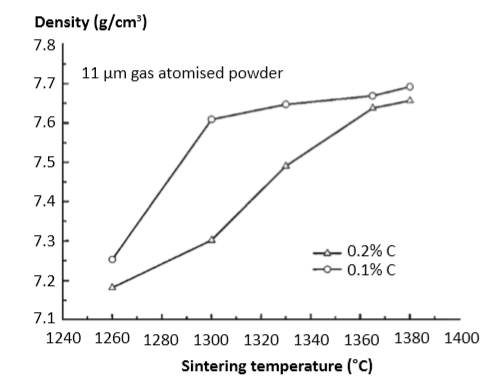
Fig. 14 Sintered density for two different carbon levels using a gas atomized 11 µm powder. Hold time at each temperature was 100 min [47] 两个不同含碳量的生坯其烧结使用气体雾化11µm 粉末,保温时间在则是100分钟[47]
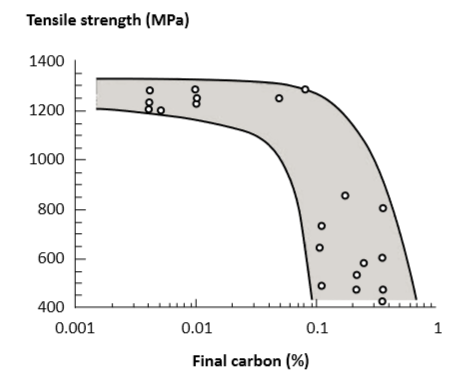
Fig. 15 Tensile strength after sintering (vacuum and hydrogen) and heat treatment, plotted versus retained carbon content [97]. This plot ignores density, grain size and other factors, so only a range of properties is associated with each carbon level 烧结后的碳含量与抗拉强度(真空和氢气)和热处理绘制成的示意图[97],这个情节忽略掉密度, 细微性和其他因素,所以只有观察一个属性就是在不同的含碳量下材料强度的表现属性。
Delta-ferrite δ-铁素体
The role of delta-ferrite (δ Fe) is mixed. On the one hand, it enhances high temperature sintering, but, on the other hand, it reduces mechanical properties. During sintering, diffusion in this body-centred cubic δ Fe phase is faster than in the face-centred cubic austenite phase, contributing to some density gains, especially for sintering temperatures below 1300°C. Longer holds or higher temperatures offset any gains from delta-ferrite formation. In hydrogen sintering, δ Fe first forms between 1190 and 1220°C [46, 111]. If the sintering atmosphere contains nitrogen, austenite is stabilised, retarding δ Fe formation and resulting in slower sintering. Yet the sintered products do not show a significant property difference when the nitrogen content is low. Also, some binders leave a carbon residue to stabilise austenite and hinder δ Fe formation. The relation between delta-ferrite δ Fe (percent) and temperature T in °C for 0.1 wt.% carbon is given as follows [111]: δ-铁素体 (δ Fe) 的作用是混合的。一方面, 它提高了高温烧结, 但另一方面, 它降低了机械性能。在烧结过程中, 这种以本体心的立方δ-铁素体相的扩散速度比以面为中心的立方奥氏体快, 有助于密度增益, 特别是在1300°C 以下的烧结温度,更长的保温或更高的温度抵消了δ-铁氧体形成的任何收益。在氢烧结, δ Fe 首先形成在1190和1220°C 之间[46, 111]。如果烧结气氛中含有氮气则可使奥氏体稳定,延缓δ-铁素体的形成,并导致烧结速度减慢。然而,当含氮量较低时,烧结产物的性能差异并不显着。此外,一些粘结剂留下了残碳则稳定奥氏体并阻碍δFe的形成。以下公式为 0.1wt%碳含量中的δ-铁(百分数)与温度T的关系, 如下[111]:
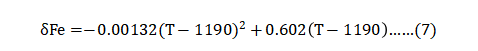
One consequence of the transformation has been mentioned, that being a peak in the sintering shrinkage rate near 1250°C after delta-ferrite forms. Retained delta-ferrite after sintering reduces the martensite content after heat treatment, resulting in lower hardness and strength. For this reason, it is important to control the δFe content. Data on the in situ behavior is thin. In a water atomized powder the initial powder has 15.5 % δFe and reaches 18.7 % after thermal de-binding. During heating to the sintering temperature, δδδδFe disappears by 750°C, reappears before 1300°C, reaches 22 % at 1360 °C, and peaks at nearly 40 % at 1380°C. δ-铁素体转变被观察到,生坯体有一个峰值收缩率发生在1250°C的烧结后,此时,δ-铁素体保留的越多便会减少热处理后的马氏体总量, 从而使产品的硬度和强度降低。因此, 控制δ-铁素体含量是很重要的,关于此原位行为的资料则是稀少的。在水雾化粉末中, 初始粉末有15.5% δ铁、在热脱粘后达到18.7%、在加热到烧结温度, δ铁会消失在750°C,又重新出现在1300°C之后,在1360°C达到22%, 和峰值在接近40%于1380°C。
On cooling, δ Fe reverts to austenite, with as little as 3% remaining after sintering [6]. Dilatometry shows about 0.3% dimensional change, beyond that due to sintering, associated with the δ Fe to austenite phase change on cooling. For gas atomized 12 µm powder, a common goal is to limit final δ Fe content to 10% or less. The map in Fig. 16 helps in matching this goal [57]. This time-temperature map is overlaid with density contours corresponding to 95.5% to 98.5% (in 0.5% increments) and delta ferrite contours. Higher sintering temperatures and longer hold times induce more δ Fe. For example, at 1300°C, up to 10% δ Fe forms in 60 min sintering [54, 57]. The plot helps identify temperature-time windows producing at least 97% density with less than 10% δ Fe, such as follows: 烧结后冷却, δ-铁素体将会恢复到奥氏体, 大约有3%剩余在量[6]。膨胀仪测定显示大约有0.3% 体积变化, 由于烧结,δ-铁素体伴生在奥氏体经冷却相变化后。对于一个采用气体雾化的12µm 粉, 一个共同的目标是将最终δ-铁素体的含量限制在10%或更少。图16中的地图有助于匹配这个目标[57]。这个时间-温度图被叠加与密度等值线对应于95.5% 到 98.5% (以0.5%为增量) 和δ-铁素体轮廓。较高的烧结温度和较长的保温时间会诱发更多的δ-铁素体。例如, 烧结于1300°C保温60分钟, 将有10%δ-铁素体的形成[54, 57]。以下在图上显示有助于识别温度时间视窗,产生至少97%密度小于10%δ-铁素体相, 如下所示:
• 1320°C for up to 20 min 1320°C保温超过20分钟
• 1300°C for 40 to 60 min 1320°C保温40~60分钟
• 1270°C for 90 to 150 min 1270°C保温90~150分钟
• 1260°C for 100 to 300 min. 1260°C保温100~300分钟
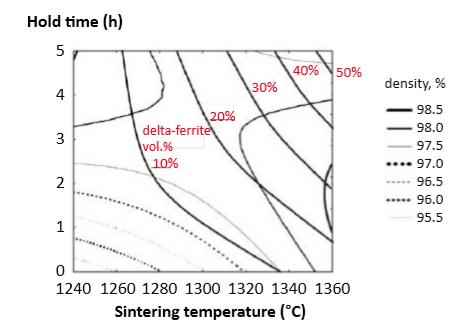
Fig. 16 Sintering time and temperature maps showing the sintered density and delta-ferrite contents using gas atomized powder [57] 使用气体雾化粉末,对应出烧结时间和温度图显示烧结密度和δ-铁素体含量[57]
No correlation is found between delta-ferrite in the sintered microstructure and final density. This means that the typical sintering hold times are sufficient to compensate for diffusion rate differences between austenite and delta-ferrite. In several cases, lower sintering temperature and longer time combinations give the highest density. Sintering in hydrogen followed by a H900 heat treatment cuts the delta-ferrite in half compared to that expected from Fig. 16 [54]. Fig. 17 plots, for both gas and water atomized powders, a comparison of delta-ferrite and residual porosity versus sintering temperature (60 min hold). The results are confusing. One study says 1300°C for 60 min delivers 10% delta-ferrite, while another study reports that over 1350°C is required. The missing parameter is the carbon content. If the target for retained delta-ferrite is 10%, then sintering a gas atomized powder in hydrogen at 1343°C results in 9.5% retained delta-ferrite [56]. 在烧结显微组织和最终密度中, δ-铁素体和其相关性几乎没有,这意味着典型的烧结保温时间足以弥补奥氏体与δ-铁素体之间的扩散速率差异。在一些情况下, 较低的烧结温度和较长的保温时间组合可以得到最终的最高密度。在氢气下烧结之后, H900 热处理会将δ-铁素体氧体的比例降低一半, 这与图16 [54]的预期可相比较。图17为气体雾化和水气雾化粉末的区块,,比较了δ-铁素体和剩余孔隙度与烧结温度 (保温均为60 分钟)。结果相当令人费解,一项研究说1300°C 保温60分钟将获得10% δ-铁素体氧体, 而另一项研究报告说, 超过1350°C 才会得到这样的结果,缺少的参数是碳含量的报告。保留的δ-铁素体的目标是 10%, 然后在氢保护下已1343°C 烧结,气体雾化粉末结果可以保留δ-铁在9.5% [56]。
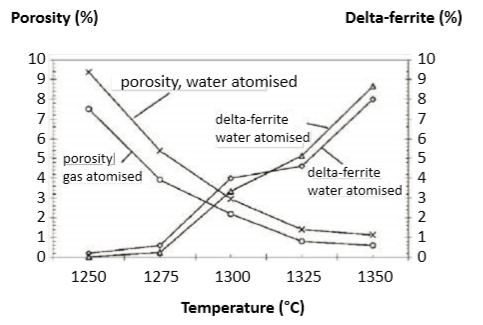
Fig. 17 Data for the sintered porosity and delta-ferrite content in heat treated 17-4 PH after sintering for 60 min at various hold temperatures in hydrogen [54]. The results include gas and water atomized powders在热处理 17-4 PH所得数据为烧结坯体的孔隙率和δ-铁素体含量比较,在不同的烧结温度以氢气保护,保温60分钟 [54]。结果包括气体和水气雾化粉末。
Hot Isostatic Pressing 热等均压
For applications sensitive to fatigue failure, a protocol is to remove lingering pores via Hot Isostatic Pressing (HIP). If sintering delivers at least 95% density, then Hot Isostatic Pressing is effective without a container; called containerless HIP. At lower densities, encapsulation is required to prevent gas intrusion into the pores. 对于耐疲劳失效敏感的应用, 一种制程是通过热等静压(HIP)去除滞留产品内部的孔隙。如果烧结能提供产品具有至少95% 密度时, 则热等静压是有效而不需要容器封装:称为无封装HIP。在较低的密度且具有开放性孔洞,则需要封装,以防止气体侵入开孔隙中。
Both gas and water atomized powders sintered in hydrogen for 60 min at 1300°C reach the required density for containerless HIP densification. Lower sintering temperatures fail to close the pores, so density is unchanged by HIP. Hot Isostatic Pressing at 1120 to 1165°C for 180 to 240 min at 103 MPa reaches essentially full density [19, 43, 112, 113]. However, tensile properties after HIP and heat treatment are little changed from that prior to HIP. For example, yield strength increases from 899 MPa to 908 MPa and fracture elongation increases from 12.2 to 14.6%. It would appear HIP is of little justification for tensile properties. 在1300°C 中保温60分钟后,用氢气烧结的气体和水气雾化粉末都达到了无容器封装HIP致密化所需的基本密度要求,在较低的烧结温度将无法获得全封闭孔洞的生坯, 所以低密度不适合于HIP后处理。热等静压在1120~1165°C保温180~240分钟保压于103 MPa,可到达完全密度[19, 43, 112, 113]。然而, HIP和热处理后的拉伸性能与HIP前的强度变化不大。例如,屈服强度从899MPa增加到 908 mpa,断裂伸长率从12.2% 增加到14.6%。看起来HIP对拉伸性能没有什么显着提升。
Besides density and tensile properties, testing after HIP involves impact toughness using subsize un-notched bars. Compared to sintered compacts, HIP increases toughness by 25 to 50%. However, impact toughness is still low at 10 J [43]. Test results from HIP full size notched Charpy bars are similar, near 10 to 11 J. Compare this with the industry standard of 140 J for un-notched subsize bars [2]. Data from another HIP study reports the tensile property gains listed in Table 7 [114]. Both materials were heat treated to H1025 (552°C for 4 hour). 除了密度和拉伸性能, HIP来处理不带缺口材料杆的半尺寸冲击韧性试片,与仅压缩烧结件相比, HIP处理后的材料韧性提高了将近25到50%。然而, 冲击韧性仍然低约在10J [43]。而以不带缺口的全尺寸冲击试片试验结果类似, 接近10~11J与工业标准的140J无缺口半尺寸条仍有段差距[2]。另一个HIP研究的资料包告了表 7 [114] 中列出的拉伸性能增益。这两种材料都H1025热处理(552°C保温4 小时)。
Table 7 Mechanical property changes with Hot Isostatic Pressing, in H1025 condition过HIP处理后的17-4PH试片之机械性质,试片以经以H1025热处理。
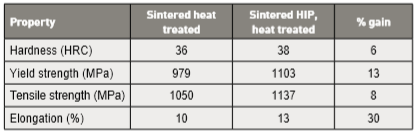
各位读者一定要清楚,17-4PH经过HIP未必可以提升太多的机械性能,除了缺口冲击韧性有明显帮助外。唯一能确定的是保障没有内部孔洞,但前提是要有封闭的孔洞,如果表面有孔洞,没有适当的处理或封装也是白做的。
Heat treatment 热处理
For strength and hardness, the desire is a martensite structure with less than 10% delta-ferrite [1]. If this is attained in the as-sintered condition, a tensile strength of 900 to 1100 MPa with 4 to 5% elongation is typical. After solutionisation (1038°C for 30 min), heat treatment to the H900 condition (482°C, 60 min) improves the tensile strength and ductility, reaching 1050 to 1100 MPa with 9% elongation. In some cases, water atomized powder is less responsive to heat treatment. Part of the effect is from how residual oxygen in water atomized powder reacts with carbon during sintering. Carbon and nitrogen influence the martensite transformation responsible for strength [45]. Unfortunately, most studies do not report final carbon, oxygen and nitrogen levels, but do report sintered density, tensile strength and ductility. 为了强度和硬度, 期望烧结后坯体保有马氏体结构且少于10% δ-铁素体[1]。如果这是在烧结条件下达到的, 拉伸强度将可在900~1100 MPa,并有4~5% 的伸长率,施以固溶处理条件 (1038°C 为30分钟) 后,再施以热处理 H900 条件(482°C, 60 分钟) 来提高抗拉强度和延性, 达到1050~1100 MPa 并有9%的伸长率。在某些情况下, 水雾化粉产品对热处理反应较少,部分的原因是从水雾化粉末中的残余氧与碳在烧结过程中的反应,碳含量便降低,碳和氮气会影响马氏体转变的能力[45]。不幸的是, 大多数研究并没有报告最终的碳、氧和氮水准, 仅报告了烧结件的密度、抗拉强度和延性。
One study examines 1300°C vacuum sintered 10 µm powder and reports a benefit from longer aging time. Peak hardness (41.5 HRC), tensile strength (1320 MPa) and ductility (10%) came with 180 min aging at 482°C [97]. This is triple the usual H900 aging time. For reference, a peak heat treated tensile strength of 1485 MPa with 9% elongation is attainable without additives [56]. Comparative heat treatment effects are summarized in Table 8 [19, 3, 115, 116]. These include different aging conditions and Hot Isostatic Pressing prior to aging. Two reports are at an elevated temperature. The tensile strength at 316°C is 689 to 772 MPa with 7 to 9% elongation. One elastic modulus report is anomalously high compared to other findings that average about 196 GPa [43, 117]. Poisson’s ratio is 0.29 for full density and changes little with density. 一项研究采1300°C以真空烧结10µm的粉末坯体, 并报告其接受较长的时效时间:原来产品坯体具有峰值硬度(41.5 HRC),抗拉强度(1320 MPa)和延性(10%),并再482°C经过180分钟时效做比较[97],这是一个三倍于通常H900 的时效时间(一般仅需要60分钟), 最后得到峰值抗拉强度1485 MPa并有9% 伸长率, 没有任何添加剂[56]。在表8 [19、3、115、116] 中比较了各种17-4PH经过热处理的效果,这些条件包括不同的时效时间和热等静压在时效之前的处理。有两份报告则是指出升温在316°C 时的抗拉强度是689~772 MPa、并具有7~9%的伸长率,弹性模量的报告是异常高出其他的发现,正常弹性模量的平均值大约196 GPa [43, 117]。泊松比是0.29 ,在全密度和变化很少的状态下。
Table 8 Heat treatment effects on sintered 17-4 PH stainless steel 不锈钢17-4PH的热处理影响烧结件的机械性质。
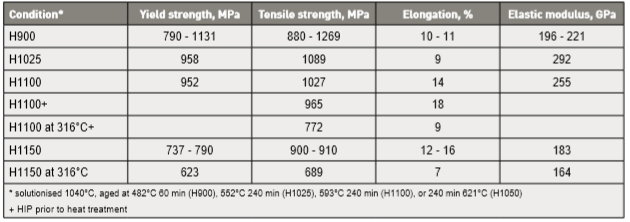
*17-4PH不锈钢必须先进行1040℃固溶处理,再加以时效于482℃, 60分钟(H900);552℃, 240分钟(H1025);593℃, 240分钟(H1100);或是621℃, 240分钟(H1050)+H1100+指的是先HIP再施加H1100处理
许多人认为17-4PH粉末烧结后跟随直接热处理就可得到好的机械性能,却常常发现做不好,鬼就在铁素体的残留阿~~在论文的做法却都是以急冷的方式冷却产品到是温取出后,再重新进行固溶处理再加上热处理,这样才是正确的。因为冷却到室温才有办法消除更多的铁素体。
Microstructure 微结构
Sintering involves a competition between densification, pore rounding, pore coarsening, grain growth and phase transformations. Pore coarsening is from Ostwald ripening, where small pores emit vacancies that migrate and coalesce into large pores. The sintered microstructure typically evidences martensite, some delta-ferrite and residual spherical pores. The pores may be filled with process atmosphere or reaction products such as steam. Retained austenite is sensitive to strain, so it decreases during a tensile test. For this reason, post-test analysis is not accurate for quantifying the relative phases. Likewise, the cooling process allows for phase changes. Quenching during sintering or studies using in-situ neutron or X-ray analysis find differences from that obtained after furnace cooling [6, 84]. The sintered microstructures also evidence spherical inclusions identified as silica-based particles, possibly chromium-silicon oxides [51, 59]. These sites nucleate ductile dimple fracture, so they are evident at a high concentration on fracture surfaces. 烧结涉及致密化、孔隙消除、孔洞粗化、晶粒生长和相变化的相互竞争,孔洞粗化是从奥斯特瓦尔德熟化现象而来, 小的孔产生空孔缺迁移和合并成大的孔洞。烧结的显微组织典型的证据是马氏体、一些δ-铁素体和残余球形孔隙。孔可能充满过程气氛或反应产品如蒸汽。残余奥氏体对应变敏感, 因此在拉伸试验中会逐渐减少。因此, 测试后分析对于量化相对结晶相是不准确的。同样的,冷却过程也允许相变化发生。在烧结过程中淬火,或使用原位中子或 X 射线分析研究可以发现显微结构与炉内冷却后获得的差异[6, 84],烧结显微组织还有证据表明,球形包裹体中有硅基粒子, 可能是铬-硅氧化物[51, 59]。这些球状物出现在脆性破的酒窝结构中的核心部位。
Grain growth during sintering results in progressive enlargement of the grains. The growth behavior is a function of sintering time and temperature, usually expressed as follows: 烧结过程中晶粒的生长导致晶粒逐渐增大,晶粒的生长行为是烧结时间和温度的函数, 通常表示如下:
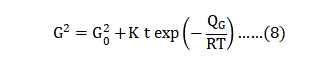
Here G is the median grain size after sintering for time t at absolute temperature T , starting at a grain size G0 , with R = gas constant 8.31 J/ (mol K), K = rate constant and QG = activation energy. Since the activation energy and rate constant are unknown for 17-4 PH, the normal protocol is to map grain size versus time and temperature. An example is given in Fig. 18 for gas atomized powder [57]. Grain growth and densification are inherently connected, since both depend on diffusion at the grain boundaries. Densification requires atomic motion along the grain boundary and grain growth requires atomic motion across the grain boundary. For this reason, the grain size G relates to the starting grain size (or particle size) GO and sintered fractional density f as follows [118]: 这里 G 是在烧结以后的中值晶粒尺寸,在绝对温度 T, 开始的晶粒大小为G0, 与R =气体常数 8.31J/(摩尔°K), K =速率常数和 QG = 活化能。17-4 PH不锈钢由于活化能和速率常数是未知的, 正常的条件下细微的反应与保温时间和加热温度有关。在图18中是使用气体雾化粉[57]是一个例子,晶粒的生长和致密化是由内在的烧结颈内所联系着, 因为两者都依赖于在晶界的扩散。致密化需要沿晶粒边界进行原子运动,晶粒生长需要跨晶粒边界的原子运动。因此,晶粒尺寸 G 涉及起始晶粒尺寸(或粉末的粒径)和烧结分数密度f如下式[118]:
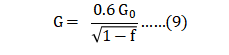
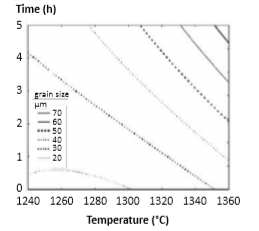
Fig. 18 Grain size map for various combinations of peak temperature and hold time [56] 各种峰值温度和保温时间组合的晶粒尺寸图[56]
The factor 0.6 compensates for the starting green density, typically near 60%. Fig. 19 plots data for 8 µm water atomized powder sintered with various combinations of time (0 to 60 min) and temperature (1120 to 1320°C), plotted as per Equation 9 [81, 82]. The starting grain size G0 is 7.6 µm, essentially the same as the particle size. 因数0.6 作为补偿起始升坯的密度, 通常接近60%。图19的资料是8µm 水雾化粉烧结件的不同时间组合(保温由0 ~60分钟)和温度(1120~1320°C)的区块数据, 按方程式9[81, 82] 绘制。起始晶粒尺寸为7.6 µm, 与粒径基本相同(一颗粉末单一晶体)。
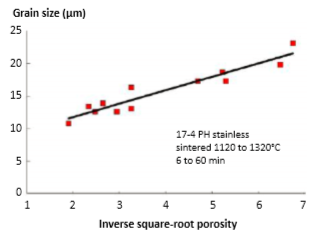
Fig. 19 Grain size versus the inverse square root of the fractional porosity (1/(1 – f)½) for a variety of sintering conditions [81,82]. The starting grain size is 7.6 µm 不同烧结条件下,晶粒尺寸与分数孔隙率的逆平方根 (1/(1-f)½)有关 [81, 82]。起始晶粒尺寸为7.6 µm。
Mechanical properties 机械性能
Already several mentions of mechanical properties have arisen. The focus on mechanical properties derives from the fact that sintered 17-4 PH is mostly used for structural devices. Strength, hardness and elongation are favorite comparative properties, already used here to examine the influence from several processing factors. Fatigue, fracture toughness, yield strength and reduction in area also are concerns, but they are reported less frequently. 有几个实验均有观察到机械性能的提升,对机械性能的关注来源于烧结17-4 PH主要用于结构装置的事实。强度、硬度和伸长率是最受欢迎的比较特性, 已经在这里使用, 并从几个加工因素的影响来检验。疲劳、断裂韧性、屈服强度和面积减少。这些都是人们关注的问题, 但报告的频率显然较少。
“The focus on mechanical properties derives from the fact that sintered 17-4 PH is mostly used for structural devices.” “对机械性能的关注来源于一个事实, 即烧结17-4 PH主要被用于结构装置。”
Many reports assess sintered mechanical properties [27, 43, 44, 47, 51-56, 60, 65, 66, 71, 77, 80-83, 92, 94, 116-127]. In addition, Additive Manufacturing and Metal Injection Molding firms provide an abundance of unaudited property reports. This summary only includes studies where the powder, processing and similar details are reported. These generally involve a wide range of powder types, with a typical 12 µm median particle size. Forming includes die compaction, injection molding, binder jetting and feedstock extrusion options. Sintering temperatures range from 900 to 1390°C (1310°C is typical), hold times range from 10 to 180 min (75 min is typical) and sintering atmosphere includes vacuum, argon-hydrogen, nitrogen, hydrogen and nitrogen-hydrogen. Almost half of the studies perform post-sintering heat treatments, usually solutionisation followed by aging at 482°C 60 min (H900). At this point, only H900 properties are included. 许多报告评估烧结件的机械性能 [27, 43, 44, 47, 51-56, 60, 65, 66, 71, 77, 80-83, 92, 94, 116-127]。此外, 增材制造和金属注塑成形公司提供了大量的未经审核的报告,本文仅摘要包括有关粉末、加工和类似细节的研究部分。这些报告中通常涉及广泛的粉末类型,以12µm 中值颗粒大小为典型的实验材料或是生产用料。成型包括压制、注塑、粘结剂喷射和喂料挤出等的选择工艺。烧结温度范围从900~1390°C (其中1310°C 是典型的), 保持时间范围从10~180分钟(75分钟是典型的), 烧结气氛包括真空、氩/氢、氮气,,纯氢以及氮/氢混合。近半数的研究进行了烧结后的热处理, 通常进行固溶后的时效在 482°C保持60分钟(H900)。因此,机械性能的报告只包含H900处理后的。
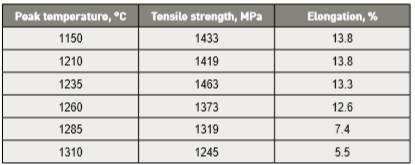
A few studies compare sintered material with wrought material after passing the wrought material through a simulated sintering cycle [51, 121]. Heating to temperatures over 1235°C (when δ Fe probably forms) weakens the wrought material with significantly less ductility, as summarized in Table 9 [121]. This property deterioration comes from grain coarsening, formation of contiguous delta-ferrite stringers on grain boundaries and element segregation between phases. At the sintering temperature, Ni and Cu diffuse into austenite while Cr and Si diffuse into delta-ferrite. Such segregation is difficult to remove by a lower temperature post-sintering heat treatment [123]; homogenization at 1150°C is required [122].通过对比烧结回圈, 烧结材料与锻料进行了比较研究[51、121]。加热到温度在1235°C (当然δ-铁素体可能形成)时烧结的17-4PH比起锻造的材料明显地也降低了延性, 总结报告在表9[121]。这一性质的恶化来自于晶粒的粗化, 形成连续的δ-铁素体在晶界和元素的分离之间的位置。在正常烧结温度下, 镍和铜会扩散到铁的奥氏体, 而铬和硅扩散成δ-铁素体。这种偏析很难通过低温后烧结热处理消除[123],必须依赖固溶均匀化在1150°C进行消除[122]。
Table 9 Wrought 17-4 PH tensile properties after heating to various sintering temperatures 锻造与烧结件17-4PH不锈钢的拉伸性能做比较数据表
Replica samples generally give low scatter; standard deviations for sintered tensile strength are reported as ± 4 to 48 MPa, elongation as ± 0.4 to 2% and hardness as ± 2 HRC. Average properties are calculated using 82 reports and the summary is given in Table 10. This includes only those reports where the sintered density is 94% of theoretical or higher. The limitation to 94% or higher density is the same as used in the industry standard [2]. The final column summarizes the properties using only results from heat treated samples. For both groups, the mean density is 97.3% (7.58 g/cm3). Statistical analysis of the studies behind these values attributes 60% of the property variation to sintering parameters, but molding, de-binding and heat treatment are also recognized factors [42]. 复制样本一般会给出低散布的数据;烧结抗拉强度的标准差报告为4~48MPa、伸长率为0.4 ~2%, 硬度值为+/-2 度HRC值。平均属性是使用82篇报告计算的, 摘要在表10中表现出来,这些只包括那些烧结密度大于94% 的理论密度或更高的报告。限制大于94%相对密度或更高的密度是与工业标准相同的[2]。最后一列也仅使用经过H900热处理的样品结果数据来汇总属性。对于两组数据, 其平均密度为 97.3%(7.58 g/cm3)。这些研究的统计分析属性有60% 的性质变化取决于:烧结参数, 但成型、脱脂和热处理也是公认的因素[42]。
Table 10 Average properties for 17-4 PH stainless steel sintered to a density of 94% or higher 烧结件17-4 PH不锈钢的平均性能烧结到94%相对密度或更高的密度
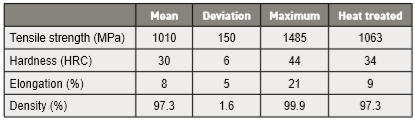
The industry standard gives a minimum tensile strength of 790 MPa and hardness of 27 HRC after sintering and 1070 MPa and 33 HRC after heat treating, both with 4% elongation. For comparison, a survey of sintered medical grade 17-4 PH (H900 heat treatment) reports density at 97.4%, hardness at 37 HRC, yield strength of 1090 MPa and tensile strength of 1196 MPa [127]. 在热处理后, 工业标准给出了烧结后最低要有 790 MPa 抗拉强度和HRC 27的硬度值以及热处理后要有1070MPa抗拉强度和HRC 33的硬度, 两者都有4% 的伸长率。为了比较, 烧结医疗等级的17-4 PH (经H900 热处理后)的调查报告密度为 97.4%, 硬度为HRC 37, 1090MPa 的屈服强度和1196MPa的抗拉强度 [127]。
Many factors influence the mechanical properties. Statistical analysis shows a dominant factor for tensile strength σU is the fractional sintered density fS , accounting for 82% of the variation:影响机械性能的因素很多。统计分析表明, 拉伸强度的一个主导因素σU 是分数烧结密度 fS, 占82% 的变化:
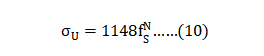
where N=5.67, similar to other sintered metals [68]. As an example, Figure 20 plots the ultimate tensile strength versus sintered density for a water atomized powder sintered 60 min in hydrogen [59, 92]. The increase in load bearing area due to densification is an important factor with respect to strength. Although sintered density is a dominant factor with respect to strength oxygen, carbon, and nitrogen play a role [44, 47, 71]. Elongation to fracture results are included in the same plot. 其中N = 5.67, 类似于其他烧结金属[68]。例如, 在图20中绘制了水雾化粉末在氢气中烧结保温60分钟的极限抗拉强度与烧结密度的关系[59、92],因致密化而增加的承重面积是强度表现好的一个重要因素,虽然烧结密度是一个主导因素关于含氧量, 以及碳和氮气扮演角色 [44, 47, 71]。断裂结果的伸长率也包括在同一报告中。
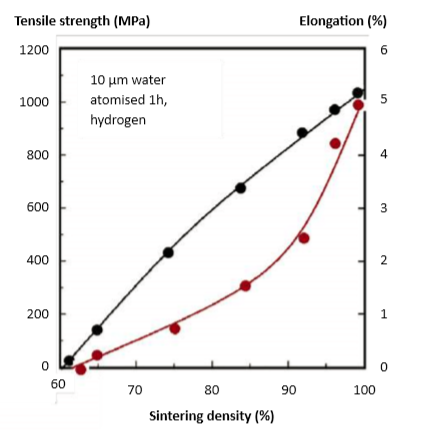
Fig. 20 Sintered strength (upper curve) and elongation (lower curve) of 10 µm water atomized 17-4 PH stainless steel powder after sintering 60 min in hydrogen at temperatures from 900 to 1350°C [59, 92] 水雾化 17-4 PH 不锈钢粉末10µm粒径的烧结强度(上曲线)和伸长率(下曲线)在从900到1350°C 的温度下, 在氢气中保温60分钟后的高温[59, 92]
Fracture toughness also varies with sintered density, ranging from 4 to 9 MPa√m for densities of 98.5 to 99.2 % [66]. As a benchmark, fracture toughness for wrought 17-4 PH is 48 MPa√m [128]. 断裂韧性也随烧结密度而变化, 范围从4~9 MPa√m, 密度为98.5~99.2% [66]。作为基准, 锻造 17-4 PH的断裂韧性为 48 MPa√m [128]。(显然烧结件比起锻造的断裂韧性差很多)
In turn, hardness directly relates to strength. The ultimate tensile strength and HRC hardness correlate as follows: 以下公式, 硬度直接关系到强度。极限抗拉强度和HRC硬度相关如下:
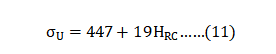
In other words, hardness is equally sensitive to those same factors that determine sintered strength and is dominated by sintering factors. The elastic modulus is reported in the 186 to 207 GPa range [2, 43, 117], except for giving values up to 292 GPa [115]. Poisson’s ratio is 0.29. 换句话说, 硬度对那些决定烧结强度和受烧结温度因素控制的因素同样敏感,弹性模数在186~ 207GPa范围[2, 43, 117], 锻造品的给定值在 292 GPa [115]。泊松比为0.29。
Fracture elongation is sensitive to fractional density and expresses that sensitivity via the β parameter in the following relation [68]: 断裂伸长率敏感于分数密度, 表示其灵敏度通过β参数在以下关系式[68]:
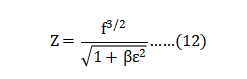
where Z is the elongation relative to full density material and ε is the fractional porosity. The ductility data in Fig. 20 correspond to β = 300, with a full density ductility of 5.2% in this case. This sensitivity to porosity is lower than observed in other sintered steels [96, 129]. 其中 Z 是相对于全密度材料的伸长率, 而ε是分数孔隙度。图20中的延性资料对应于β = 300, 在这种情况下, 全密度延性为5.2%。这种对孔隙度的敏感性低于其他烧结钢材[96, 129]。
Impact toughness evidences a high variability that depends on test conditions [43, 103, 116, 120]. There are at least four tests in use, notched and un-notched, and full size (10 by 10 mm) and subsize (5 by 10 mm). The standard Charpy test relies on a 2 mm deep notch in a full size bar. As evident in Table 11, the findings are highly scattered [5, 43, 103, 116, 120, 128]. This compilation includes comparative data for cast and wrought 17-4 PH. The heat treated condition is H900 and a few tests are in the solutionised condition. For comparison, the industry sets 140 J as the recommended value for subsize un-notched test bars, both as-sintered and heat treated. No test data are published to support this value, so caution is advised for applications sensitive to impact loading. Testing in use temperature, strain rate and surface finish conditions is advised to avoid surprises. 冲击韧性证明了取决于测试条件的高变异性[43, 103, 116, 120]。至少有四项试验分别使用缺口、未缺口、全尺寸(10对10毫米)和半尺寸(5对10毫米)。标准的摆锤测试,依赖于一个具有2毫米深凹槽在一个全尺寸的测试棒。如表11所示,调查结果呈现高度分散数据[5, 43, 103, 116, 120, 128]。此汇编包括用于铸件和锻件的17-4 PH一起比较资料。热处理条件都以H900实施,在固溶条件下进行了一些试验。为了比较, 工业标准以设置 140 J 作为建议的半尺寸未缺口试验棒的冲击耐受能耐, 无论是烧结件和热处理件。但是没有一份报报公开这数值, 因此建议对设计和使用者标注耐冲击使用的警告。建议在使用温度、应变率和表面光洁度条件下进行测试,避免出现意外。(读者们要注意和客户协议耐冲击的测试标准,很重要!!)
Table 11 Comparative impact toughness reports冲击韧性比较报告
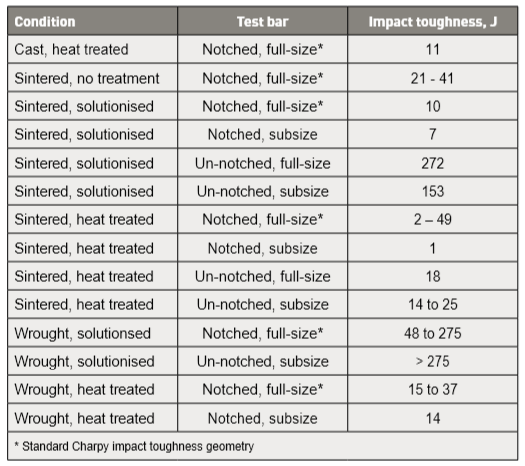
Residual pores cause low impact toughness, but second phases (delta-ferrite), segregation of impurities and inclusions are problems. Although the industry standard is 140 J, the sintered results are much lower, tending toward 20 J (H900). Higher aging temperatures improve the response, reaching up to 50 J for notched full-size bars aged at 610°C [116]. Like tensile tests, the fracture surface shows evidence of delta ferrite and ductile dimples nucleated at silica or chromic inclusions [39, 51, 130]. 残余孔隙造成冲击韧性降低, 第二相(δ-铁素体)、杂质和夹杂物都是问题原因之一。虽然工业标准是140 J, 众多报告的烧结件测试结果是低得很多, 倾向于20 J (H900)。较高的时效温度改善了这个结果网好走,缺口全尺寸试棒可达到50 J在 610°C时效处理后 [116]。与拉伸试验一样, 断口显示了δ-铁素体和韧性酒窝中有二氧化硅或铬包裹体的核状物证据 [39, 51, 130]。
Reports on the fatigue endurance limit are collected in Table 12 [43, 49, 114, 130]. Fatigue strength for the fully reversed cycle (R = -1, where R = min stress over max stress) at 50% survival is 517 MPa for sintered mixed elemental powder heat treated to H900. On the other hand, gas atomized powder in the H1150 heat treatment is lower at 423 MPa. Due to scatter, the 99% survival fatigue strength is reduced to 362 MPa. Other reports compare heat treatment against as-sintered, giving 630 and 470 MPa, respectively. Low cycle fatigue after HIP and heat treatment (H1100) is not as good as wrought, but better than cast material [19]. Over a large number of studies, there is no statistical evidence of mechanical property differences between gas and water atomized powders. However, in a direct comparison, the tensile strength is 1280 MPa for gas atomized and 1136 MPa for water atomized after sintering 1350°C for 60 min followed by solutionisation and H900 heat treatment [53]. Note, heat treated tensile strength reaches to 1485 MPa with 8-9% elongation using gas atomized powder [44, 56, 64]. Likewise, for ductility, a direct comparison for 1300°C sintering using 14 µm gas and water atomized powders reports 14.5% elongation and 1% elongation, respectively [71]. The sintering cycle apparently needs to be modified to accommodate the powder type and then similar properties are possible. 在表 12 [43, 49, 114, 130]中收集疲劳耐力极限的报告,完全反转回圈的疲劳强度 (R =-1, 其中R=最小应力超过最大应力) 在50% 存活率是517MPa,烧结混合元素粉末热处理H900。另一方面,气体雾化粉末在H1150热处理后低于423兆帕。由于分散效果,99% 的生存疲劳强度减少到362兆帕。其他报告比较了热处理与烧结, 分别得到630~470MPa。HIP和热处理后的低周期疲劳(H1100)不如锻件好, 但比铸件材料[19]要好。在大量的研究中,没有统计证据表明气体和水雾化粉末之间的力学性能差异。然而,直接比较,抗拉强度是气体雾化为1280 MP和水雾化为1136 MPa , 烧结1350°C保温60分钟后,在施以固溶和 H900热处理[53]。注意,热处理后抗拉强度达到1485MPa,延伸率8-9%,使用气体雾化粉末[44, 56, 64]可达到这样的数据。同样对于延性而言,直接比较于1300°C 烧结使用14µm 气体和水雾化粉末报告得到分别为14.5%的伸长率和1% 的伸长率,[71]。烧结周期显然需要修改,以适应粉末类型,然后才可能获得相类似的性质。
Table 12 Fatigue endurance strength (107 cycles) for sintered 17-4 PH stainless steel烧结 17-4 PH 不锈钢的疲劳耐久性强度(107个周期)
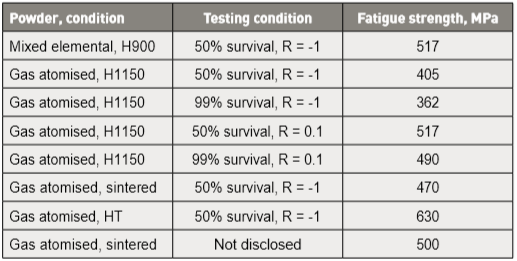
Corrosion, wear and biocompatibility 腐蚀、磨耗和生物相容性
Standard corrosion testing involves exposure to various environments - ferric chloride, artificial saliva, chloride bleach, salt water, Ringer’s solution, dilute sulphuric acid, copper sulphate solution, nitric acid and salt sprays [2, 51, 95, 131-135]. Extensive corrosion testing of four sintered stainless steels found 17-4 PH to be least resistant to a variety of environments [134]. Wrought material is more corrosion resistant than sintered material, partly because pores induce local corrosion (concentration polarisation) in the sintered material. However, when wrought is subjected to a “sintering” cycle it too degrades. The high temperature exposure associated with sintering induces segregation that gives galvanic corrosion. Also, any retained oxygen is a negative factor. Oxygen is avoided by use of gas atomized powder [135]. However, in one test, sintered (1370°, 75 min, hydrogen) master alloy powder resulted in a better corrosion resistance (2% salt water, 60°C, 2 weeks immersion) [70]. 标准腐蚀测试包括接触各种环境-氯化铁、人工唾液、氯化物漂白剂、盐水、复方氯化钠(林格氏液体)、稀硫酸、硫酸铜溶液、硝酸和盐雾实验[2, 51, 95, 131-135],四个烧结件不锈钢的广泛腐蚀试验发现, 烧结件17-4PH对各种环境的抵抗力都低[134],锻件比烧结件具有更耐腐蚀性, 部分是由于孔隙引起烧结件局部腐蚀(浓度极化反应)。然而, 当锻件采用 "烧结" 周期来处理后, 它们也会退化,因为烧结有关的高温暴露会导致材料引起偏析, 从而产生电偶腐蚀。此外, 材料有任何保留的氧气是一个负面的因素,使用气体雾化的粉末有较低的残留氧气[135]。然而, 在一次试验中, 烧结(1370°C,保温75 分钟, 氢)母合金粉末导致更好的耐腐蚀性(2% 盐水, 60°c, 2 周浸泡)[70]。
Generally, hydrogen sintering provides corrosion resistance. In a novel study, 14 µm gas atomized powder (sintered in hydrogen for 60 min at 1300°C) has a low corrosion rate (1.7 g/(m2 h)) in 6% ferric chloride solution when solutionisation is skipped and aging extended to 180 min at 480°C [27]. As-sintered salt water immersion tests report about 2.2 g/ (m2 h) corrosion rate [88]. Table 13 illustrates the differing corrosion rates [27]. The sintered material (1300°C, 60 min, H2) without solutionisation, but with 120 min aging at 480°C, was the most corrosion resistant. A different study (water atomized, sintered 1340°C, hydrogen, 30 min) reports that sulphuric acid corrosion resistance was best in the H900 condition [136]. Adding to the confusion, one report is for corrosion resistance after sintering without solutionisation or aging [95]. For medical applications, nitric acid passivation reportedly improves corrosion resistance [127]. No indication is given on how frequently this is applied or the corresponding rate of corrosion. 通常来说,氢烧结提供较好的耐腐蚀性能,在一项新的研究中,以14µm气体雾化粉末(1300°C保温60分钟的氢烧结)有低腐蚀率(1.7 克/(m2•h)) 在6% 氯化铁溶液时,固溶处理被跳过和但在480°C时效延长到180分钟[27],烧结件经盐水浸泡试验报告值约2.2克/(m2•h)的腐蚀速率[88]。表13说明了不同条件下的腐蚀速率[27]。烧结件材(1300°C,保温60分钟, H2) 没有经过固溶处理、但在480°C 时效了120分钟是最耐腐蚀的。另一项研究 (水雾化, 烧结在1340°C,氢气,保温30 分钟)报告说明,耐硫酸腐蚀性最好是以H900处理后的烧结件[136]。令人混淆的是,有一份报告是为腐蚀抵抗在没有经过固溶或时效的烧结件上[95]。对于医疗应用, 据报告硝酸钝化可改善耐烧结件的腐蚀性[127]。但没有任何数据表明, 这样的处理可以耐受多久。
Table 13 Ferric chloride (6% solution) corrosion rates氯化铁(6%溶液)腐蚀速率
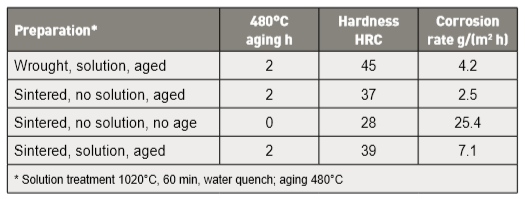
Wear tests on sintered 17-4 PH using the pin on disc test show that material loss depends on sintered density, as illustrated in Fig. 21 [66]. The mass loss increases with sliding distance and load. No comparative standards are included, so the relative wear behavior of sintered versus wrought material is unknown. 用针状试片在圆盘上的磨损试验中对烧结 17-4 PH 进行测试,发现材料损耗取决于烧结件密度,如图 21 [66] 所示。随着滑动距离和载荷的增加,试片重量损失增大。在不包含比较标准下, 烧结件与锻件的相对磨损行为是没有比较数据的。
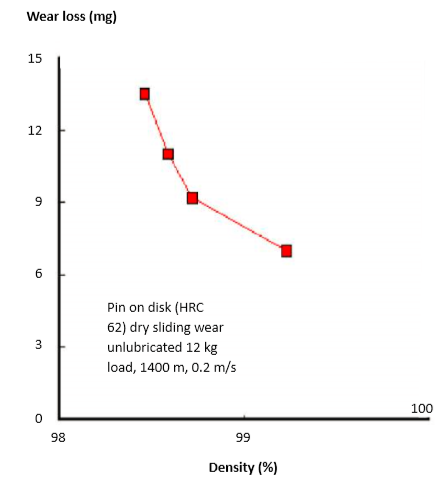
Fig. 21 Wear loss in sliding pin on disc tests for sintered 17-4 PH, illustrating the gains in wear resistance that come with higher sintered density [66] 以17-4PH不锈钢针状试片在圆盘上进行的磨损试验,密度越高的烧结件能够有效的阻止磨耗的发生[66]
Biocompatibility is related to corrosion resistance. The corrosion resistance makes 17-4 PH good in cytotoxicity [137], but it is not competitive in overall biocompatibility when compared to sintered titanium and cobalt alloys [138]. 生物相容性与耐腐蚀有关。尽管17-4 PH耐蚀性良好使其降低对细胞毒性 [137], 但和烧结钛和钴合金相比,整体生物相容性仍旧不足[138]。
Dimensional control 尺寸控制
Dimensional variability depends on several factors and is often dominated by shaping and feedstock factors. Sintering amplifies the effects from variations occurring during shaping. Effectively, some of the sintered size variation is embedded in the part mass variation, emerging in the sintering step [139]. Depending on sensors and controls employed in shaping, up to a ten-fold difference in dimensional variability results between vendors. The nominal capability is termed the coefficient of variation CV . Expressed as a percent, CV is measured using several components: 尺寸变异性取决于几个因素, 主要是由成形参数和喂料因素、烧结放大的影响,这些变化都发生在成型条件。有效地, 一些烧结件尺寸的变化会因为产品的重量变化造成, 出现在成形之后的烧结步骤控制[139]。根据在成形中使用的感测器和控制发现,在不同的供应商之间,可测达十倍的尺寸变异性差异。标称能力称为变异 CV 系数。以百分比表示, CV 使用多个元件进行测量:
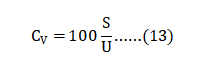
where S is the standard deviation and U is the mean size. Over the industry, the typical value is ± 0.3% [2, 131]. An assumption is that the mean size is centred to the middle of the tolerance range, which is not always possible. 其中S为标准差、U则是粉末平均粒径大小,MIM在工业上的应用典型的允差是± 0.3% [2, 131],并假设粒径平均大小集中到可容忍范围的中间, 不过总是不可能都是这样的。
An audit of injection molded and sintered 17-4 PH dental brackets finds CV ranges from ± 1.2% to ± 0.3% [137]. This is the same as seen in another study [57]. Of that variation, 69% is related to sintering time, temperature and heating rate while 31% is due to other factors. Impressive results are reported using solvent de-binding with the largest CV (± 0.17%) aligned with gravity [29]. 我们审视17-4PH制作的牙科支架的注射成形以及烧结发现CV值约在± 1.2%到± 0.3%之间变化[137],这样的现象也被另一组实验观察到[57],根据观察这变化,有69%与烧结的保温时间、保温时间和升温速率有关,其余因素占31%。令人印象深刻的结果是使用溶剂脱粘与最大的 CV (0.17%)是受到重力 的影响[29]。
Sintered dimensional prediction is a major focus of computer simulations [18, 54, 81, 82, 86]. The models require several input parameters. Most efforts invoke viscous approximations for sintering shrinkage and embed those approximations into finite element analysis. Fig. 22 plots experimental and simulated shrinkage for a 10 µm gas atomised powder. The sintering starts with 55% density, following a cycle of 10°C/ min to 1010°C with 60 min hold, then 2°C/ min to 1365°C (no hold), with cooling at 10°C/min [82]. The experimental curve is from dilatometry. The simulation prediction is off by 0.6% on final size. Such an error is larger than a typical component size tolerance. Thus, such models help in estimating size change, but not designing tooling. 用电脑程式来预测烧结件尺寸是未来的主要重点[18、54、81、82、86],可以节省大量的模具修正时间,烧结件模型需要多个输入参数。大多数的努力调用粘性近似的烧结收缩和嵌入这些近似到有限元分析程序中,如图 22以10µm 气体雾化粉的试验和模拟进行收缩预测比对。烧结开始在55%生坯密度, 跟随一个周期升温速率为10°C/分钟到1010°C 并保温60分钟来进行, 在加温到1365°C (不保温), 以冷却速率在10°C/分钟下降[82]。实验曲线来自热膨胀仪的纪录。模拟预测在最终尺寸上有0.6%的变异,此误差大于典型的物件设定公差。因此, 这些模型有助于估计烧结件的尺寸大小变化, 但不是用来设计模具。
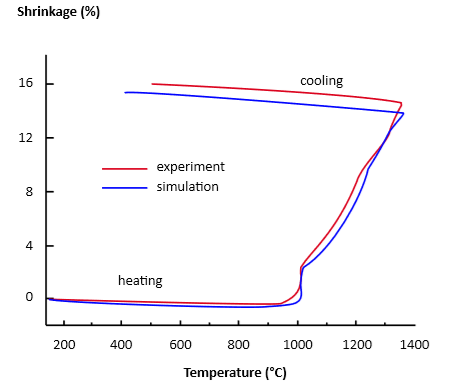
Fig. 22 Dilatometry sintering shrinkage for 10 µm 17-4 PH stainless steel powder, including both heating and cooling, comparing the computer simulated dimensional change with the experimentally measured behavior [82] 热膨胀仪测量粉末粒径为10µ的17-4PH烧结件的收缩性,图上两条曲线为实际实验和模拟对比(蓝色是模拟、红色是实验实测),在升温和降温过程都进行测量,观察两个条件下烧结件改变的行为[82]
Another approach to predicting final size is via the master sintering curve. This employs a thermal work of sintering concept as captured in an integral of the time ( t ) and temperature ( T , absolute temperature) [39, 54, 89, 111, 140]. The work of sintering θ term is calculated using an apparent activation energy QE as follows: 另一种预测最终尺寸的方法是通过主烧结曲线。这使用了烧结概念的热工作在时间 (t) 和温度 (T, 绝对温度) 的积分中被获得 [39, 54, 89, 111, 140]。用表面活化能量化法计算烧结的总功θ情况如下:
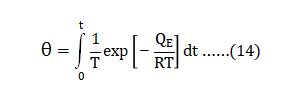
again R is the universal gas constant. The heating cycle is tracked to include the thermal contributions on the way to the peak temperature, an important part of densification. 其中 R 是通用气体常数,加热回圈的跟踪包括热贡献的方式到峰值温度,这是烧结件致密化的一个重要的部分。
The only adjustable parameter is the activation energy, which changes due to phase transformations for 17-4 PH stainless steel. The fractional sinter density fS at any point is calculated from the integral work and starting green density fG ,唯一可调节的参数是活化能, 这改变来自于17-4PH不锈钢的相变化。由积分总功θ和起始于生坯密度fG的方法计算出任意点的烧结件分数密度fS。
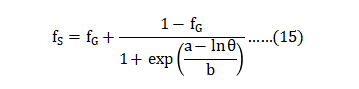
This sigmoid function accounts for how density reaches saturation at 100%, no matter what additional work is added. For 10 µm gas atomized 17-4 PH powder, value of a = 29.93 and b = 1.521 with QE = 350 kJ/mol. Equation 15 is employed to compute shrinkage and final component size. If the phase transformation is ignored, QE = 360 kJ/mol and predicted sintered size is good to within 0.8%. Smaller powders sinter more easily, resulting in a further reduction in activation energy [39] as summarized in Table 14. The model accuracy is improved by using an activation energy of 321 kJ/ mol for austenite (below 1200°C) and 350 kJ/mol for delta- ferrite. This effectively embeds the phase change into the shrinkage behavior. The two-step treatment reduces the prediction error to less than the measurement error [54, 111]. 这个S型函数说明了烧结件密度如在100% 时, 不管增加什么额外的功都是没有用的,以10µm气体雾化 17-4 PH不锈钢粉末,式中的a = 29.93和b = 1.521与QE = 350 J/mol,在方程式(15)中用于计算烧结件收缩和最终零件尺寸。如果相变化是被忽略的,QE = 360 J/mol和预测烧结件尺寸是有效的,误差在0.8%以内。更细的粉末进行烧结更容易, 因为其活化能可以进一步减少[39],如表14总结。通过使用321 J/mol的活化能(低于1200°C)和350 J/mol的δ-铁素体, 可以提高了模型计算的精度,这有效地将相变化嵌入到烧结件的收缩行为中。两步处理将预测误差降低到小于测量误差内[54、111]。
Table 14 Master sintering curve apparent activation energy versus median particle size 主烧结曲线表面活化能与粉末中值粒径关系
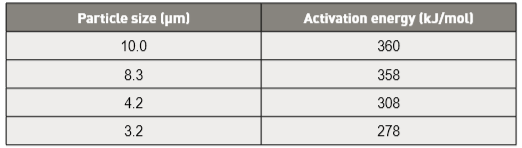
Conclusions and identified needs 结论与更多论证需求
Starting around 1990, considerable effort emerged on the sintering of 17-4 PH stainless steel, with much focus on structural property optimization. Tremendous progress has taken place, delivering an attractive combination of sintered properties suitable for many applications. 从1990左右开始,世人对17-4PH不锈钢的烧结研究投入了相当大的努力,重点是结构性能优化。现在已经取得了巨大的进步,提供了很优异的烧结性能适合多种应用的,让17-4PH变成是一个很有吸引力的材料。
With regard to properties, the sintering temperature is a dominant factor. It is more important than powder type, particle size, green density, hold time and other parameters, as covered in this article. Sintered properties are sensitive to what phases exist in the sintered material. Likewise, properties vary with the phase morphology, sintered density and post-sintering heat treatment. Hot Isostatic Pressing improves properties, but sintered density is the key factor determining properties. 在烧结性能方面,烧结温度是主要因素,它比粉末类型、粒径、生坯密度、保温时间等参数更为重要。烧结性能对烧结材料中存在的相敏感,使得相形态、烧结密度和后烧结热处理等性能也不同。热等静压固然能更提高性能,但烧结后的密度才是决定性能的关键因素。
Statistically significant correlations are found between strength and density, strength and hardness and ductility and density. However, impact properties are very suspect. The reason for variability is not clear. This implies that uncontrolled factors are causing property variations. Most likely the final carbon, oxygen and nitrogen levels, as well as phase morphology (for example delta-ferrite on grain boundaries), pore size and grain size are uncontrolled factors. Often only the starting powder carbon content is reported. Critical study is needed on final C, O, N contents as linked to properties. Further, classic heat treatments are not optimal for sintered 17-4 PH stainless steel. To emphasize this point, note that the highest tensile strength reports are associated with 17-4 PH stainless steel powders treated with intentional additives of boron or carbon. The initial alloy composition and heat treatments used for sintered powders were optimized for cast and wrought products. Sintered products benefit from modified compositions and different heat treatments, as noted in this report. 在强度和密度、强度和硬度、延展性和密度之间存在统计上显着的相关性。然而,冲击性能是非常可疑的,变异的原因目前尚不清楚。这意味着不受控制的因素导致耐冲击性质的变化,最有可能的是最终的碳、氧和氮水准,以及相形态(例如在晶界上的δ-铁素体)、孔径和晶粒尺寸是不受控制的因素。通常报告只对起始粉末碳含量控制,最后的C,O,N含量与性质相关的关键研究是有更多的需要。此外,经典的热处理对于烧结17-4PH不锈钢来说并不是最佳的。为了强调这一点,请注意,最高的抗拉强度报告与17-4PH不锈钢粉末用硼或碳的有意添加剂处理而来,对烧结粉末的初始合金成分和热处理参考了传统铸造和锻造产品并加以优化,烧结制品变得益于改进的组成物和不同的热处理,恰如本报告所述。
Two areas of uncertainty are impact toughness and corrosion resistance. Further, fracture toughness is missing adequate attention. The measures to date indicate a low resistance to crack propagation. 两个不确定区域是冲击韧性和耐腐蚀性。此外,断裂韧性缺乏足够的关注,迄今为止的措施表明17-4PH具有较低裂纹扩展阻止能力。(最好不用来做为需要耐疲劳元件?有待观察)
Much progress has taken place over the years since sintered 17-4 PH stainless steel gained attention. When an injection molded aerospace component with a tensile strength of 1068 MPa was given “part of the year” designation, sintered 17-4 PH jumped to the forefront of metal Powder Injection Molding. That platform is now an important basis for Additive Manufacturing and other binder-assisted forming approaches, all relying on this strong technology base. Much progress is evident in the many reports to date. With focused attention to some opportunities, many more applications for sintered 17-4 PH are anticipated. 多年来,烧结17-4PH不锈钢已经得到了广泛的关注。当注射成形制作的航空航天部件具有抗拉强度为1068 MPa时,被称为可用“整年份的零件”的名称(表示可以使用一整年不需要替换),烧结17-4PH不锈钢已经跃进到了金属粉末注射成形的最前沿。此技术平台现在已经是增材制造和其他粘合剂辅助成型方法的重要基础,它们都依赖于这个强大的技术基础出发,迄今为止的许多报告都有提到此材料的重要性。随着关注的一些机会,预计烧结17-4 PH的应用范围会更加扩大。
References 参考文献(不翻译)
1. D. Peckner, I. M. Bernstein, Handbook of Stainless Steels , McGraw-Hill, New York, NY, 1977.
2. Materials Standards for Metal Injection Molded Parts , MPIF Standard 35, Metal Powder Industries Federation, Princeton, NJ, 2016.
3. R. M. German, “The Production of Stainless Steels by Injection Molding Water Atomized Prealloy Powders,” Journal of Injection Molding Technology , 1997, vol. 1, pp. 171-180.
4. J. R. Davis (ed.), Stainless Steels , ASM International, Materials Park, OH, 1994.
5. H. J. Rack, Physical and Mechanical Properties of Cast 17-4 PH Stainless Steel , Report SAND80-2302, Sandia National Laboratories, Albuquerque, NM, 1981.
6. D. J. Gossens, R. E. Whitfield, A. J. Studer, “Optimising Sintering in Metal Injection Moulding Using In Situ Neutron Diffraction,” Materials Science Forum , 2012, vol. 706, pp. 1737-1742.
7. J. A. Grohowski, PIM Tolerance Control for Different Powder Systems , PM Lab, Penn State University, State College, PA, 20 Oct 1994.
8. Y. Kankawa, K. Saitou, T. Kida, K. Ono, Y. Kaneko, “Injection Molding of SUS316L Powder with Polyacetal,” J ournal of the Japan Society of Powder and Powder Metallurgy , 1993, vol. 40, pp. 379-383.
9. H. Abolhasani, N. Muhamad, “A New Starch-Based Binder for Metal Injection Molding,” Journal of Materials Processing Technology , 2010, vol. 210, pp. 961-968.
10. Y. Kankawa, M. Atarashi, Y. Akifusa, Y. Kaneko, A. Fusamoto, H. Ikeda, “Injection Molding of SUS316L Powder with Polyacetal Polyethylene Polymer-Alloy Polymer,” J ournal of the Japan Society of Powder and Powder Metallurgy , 1996, vol. 43, pp. 840-845.
11. J. Ryuhgoh, K. Kawamoto, T. Osanaga, Y. Nakata, Moldable Composition, Process for Producing Sintered Body Therefrom and Products from Same , U. S. Patent 5,432,224, issued 11 July 1995.
12. M. Achikita, A. Oktsuka, Composition for Injection Molding , U. S. Patent 5,030,677, issued 9 July 1991.
13. T. Shimizu, S. Mochizuki, T. Sano, S. Fuchizawa, “Supercritical CO2 De-binding Method in MIM Process - De-binding Conditions and Process in Supercritical De-binding,” Journal of the Japan Society of Powder and Powder Metallurgy , 1996, vol. 43, pp. 1188-1192.
14. H. H. Angermann, O. O. Van Der Biest, “Low Temperature De-binding Kinetics of Two-Component Model Systems,” International Journal of Powder Metallurgy , 1993, vol. 29, pp. 239-250.
15. K. C. Hsu, C. C. Lin, G. M. Lo, “Effect of Wax Composition on Injection Moulding of 304L Stainless Steel Powder,” Powder Metallurgy , 1994, vol. 37, pp. 272-276.
16. Y. Kankawa, “Effects of Polymer Decomposition Behavior on Thermal De-binding Process in Metal Injection Molding,” Materials and Manufacturing Processes , 1997, vol. 12, pp. 681-690.
17. H. Nakayama, H. Kyogoku, S. Komatsu, “Effect of MIM Process Conditions on Microstructure and Mechanical Properties of Sintered SUS630 Compacts,” Journal of the Japan Society of Powder and Powder Metallurgy , 1997, vol. 44, pp. 1019-1023.
18. S. Ahn, S. J. Park, S. Lee, S. V. Atre, R. M. German, “Effect of Powders and Binders on Material Properties and Molding Parameters in Iron and Stainless Steel Powder Injection Molding Process,” Powder Technology , 2009, vol. 193, pp. 162-169.
19. K. A. Green, “PIM 17-4 PH Actuator Arm for Aerospace Applications,” Advances in Powder Metallurgy and Particulate Materials - 1998 , Metal Powder Industries Federation, Princeton, NJ, 1998, pp. 5.119-5.130.
20. J. C. Lasalle, M. Zedalis, “Net-Shape Processing Using an Aqueous-Based MIM Binder,” Journal of Metals (JOM) , 1999, vol. 51, no. 7, pp. 38-39.
21. H. I. Bakan, Y. Jumadi, P. F. Messer, H. A. Davies, B. Ellis, “Study of Processing Parameters for MIM Feedstock Based on Composite PEG-PMMA Binder,” Powder Metallurgy , 1998, vol. 41, pp. 289-291.
22. T. Shimizu, Y. Murakosi, T. Sano, “Evaluations of Binder Systems for Supercritical Carbon Dioxide De-binding Process,” Proceedings 1998 PM World Congress , Granada Spain, published on CD by European Powder Metallurgy Association, Shrewsbury, UK, December 1998.
23. N. Myers, R. M. German, “Binder Selection for PIM Water Atomized 316L,” Advances in Powder Metallurgy and Particulate Materials - 2001 , Metal Powder Industries Federation, Princeton, NJ, 2001, pp. 4.27-4.34.
24. Z. Y. Liu, N. H. Loh, S. B. Tor, K. A. Khor, Y. Murakoshi, R. Maeda, “Binder System for Micropowder Injection Molding,” Materials Letters , 2001, vol. 48, pp. 31-38.
25. S. Supriadi, E. R. Baek, C. J. Choi, B. T. Lee, “Binder System for STS 316 Nanopowder Feedstocks in Micro-Metal Injection Molding,” Journal of Materials Processing Technology , 2007, vol. 188, pp. 270-273.
26. R. E. Wiech, Method for Removing Binder from a Green Body , U. S. Patent 4,404,166, 13 September 1983.
27. H. Zhang, R. M. German, “Powder Injection Molding of 17-4 PH Stainless Steel,” Powder Injection Molding Symposium 1992 , P. H. Booker, J. Gaspervich and R. M. German (eds.), Metal Powder Industries Federation, Princeton, NJ, 1992, pp. 219-227.
28. R. Ibrahim, M. Azmirruddin, M. Muhamad, R. Awang, S. Muhamad, “Injection Molding of 316L Stainless Steel Powder for Biomedical Application Using Palm Oil Derivatives Binder System,” Advances in Powder Metallurgy and Particulate Materials - 2009 , Metal Powder Industries Federation, Princeton, NJ, 2009, pp. 4.52-4.59.
29. M. S. Park, J. K. Kim, S. Ahn, H. J. Sung, “Water Soluble Binder of Cellulose Acetate Butyrate / Poly(ethylene glycol) Blend for Powder Injection Molding,” Journal of Materials Science , 2001, vol. 36, pp. 5531-5536.
30. M. H. Ismail, M. A. Omar, N. Muhamad, A. Jumahat, “Study of Evolution of Pore Structure during Water Leaching using PEG-PMMA Binder System,” Advances in Powder Metallurgy and Particulate Materials - 2005 , Metal Powder Industries Federation, Princeton, NJ, 2005, pp. 4.62-4.71.
31. M. Song, M. S. Park, J. K. Kim, I. B. Cho, K. H. Kim, H. J. Sung, S. Ahn, “Water Soluble Binder with High Flexural Modulus for Powder Injection Molding,” Journal of Materials Science , 2005, vol. 40, pp. 1105-1109.
32. Y. L. Fan, K. S. Hwang, “Properties of Metal Injection Molded Products Using Titanate-Containing Binders,” Materials Transactions , 2007, vol. 48, pp. 544-549.
33. Y. C. Kim, S. Lee, S. Ahn, N.J. Kim, “Application of Metal Injection Molding Process to Fabrication of Bulk Parts of TiAl Intermetallic,” Journal of Materials Science , 2007, vol. 42, pp. 2048-2053.
34. M. Y. Anwar, H. A. Davies, “A Comparative Review of Various PIM Binder Systems,” Advances in Powder Metallurgy and Particulate Materials - 2007 , Metal Powder Industries Federation, Princeton, NJ, 2007, pp. 4.8-4.20.
35. C. Lall, The Science of Stainless Steels Produced by Powder Metallurgy and Metal Injection Molding , Metal Powder Industries Federation, Princeton, NJ, 2006.
36. R. Raza, F. Ahmad, M. A. Omar, R. M. German, A. S. Muhsan, “Defect Analysis of 316L SS during the Powder Injection Moulding Process,” Defect and Diffusion Forum , 2012, vol. 329, pp. 35-43.
37. Y. Li, B. Huang, S. Li, “Evolution of Microstructure and Dimension of As Molded Parts During Thermal Removal Process of Wax Based MIM Binder,” Transactions of the Nonferrous Metals Society of China , 2001, vol. 11, pp. 340-344.
38. P. Setasuwon, A. Bunchavimonchet, S. Danchaivijit, “The effects of binder components in wax / oil systems for metal injection molding,” Journal of Materials Processing Technology , 2008, vol. 196, pp. 94-100.
39. D. Y. Park, G. M. Lee, Y. S. Kwon, Y. J. Oh, S. Lee, M. S. Jeong, S. J. Park, “Investigation of powder size effects on sintering of powder injection moulded 17-4 PH stainless steel,” Powder Metallurgy , 2017, vol. 60, pp. 139-148.
40. V. Demers, M. M. Elmajdoubi, P. Bocher, “Moldability of Low Pressure Powder Injection Molding Feedstocks,” Advances in Powder Metallurgy and Particulate Materials - 2017 , Metal Powder Industries Federation, Princeton, NJ, 2017, pp. 401-414.
41. M. T. Zaky, “Effect of Solvent De-binding Variables on the Shape Maintenance of Green Molded Bodies,” Journal of Materials Science , 2004, vol. 39, pp. 3397-3402.
42. J. A. Sago, J. W. Newkirk, G. M. Grasel, “The Effects of MIM Processing Control Parameters on Mechanical Properties,” Advances in Powder Metallurgy and Particulate Materials - 2003 , Part 8, Metal Powder Industries Federation, Princeton, NJ, 2003, pp. 205-216.
43. A. Luo, J. Arrecoechea, R. Wengler, G. A. Gegel, R. Carlson, D. Dufour, “Mechanical Properties of Metal Injection Molded (MIM) 17-4 PH Stainless Steel,” Advances in Powder Metallurgy and Particulate Materials - 2003 , Part 8, Metal Powder Industries Federation, Princeton, NJ, 2003, pp. 245-253.
44. D. A. Hauser, The Effects of Atmospheric Nitrogen on the Mechanical Properties of Sintered 17-4 PH Stainless Steel , MS Thesis, Engineering Mechanics Department, Pennsylvania State University, University Park, PA, May 2002.
45. D. T. Whychell, J. Stevenson, J. Peltier, M. Goldenberg, J. Lasalle, “Thermal Debind Studies on a Water Based Gel System in Stainless Steel 17-4 PH,” Advances in Powder Metallurgy and Particulate Materials - 2002 , Metal Powder Industries Federation, Princeton, NJ, 2002, pp. 10.129-10.136.
46. Y. Wu, D. Blaine, B. Marx, C. Schlaefer, R. M. German, “Sintering Densification and Microstructural Evolution of Injection Molding Grade 17-4 PH Stainless Steel Powder,” Metallurgical and Materials Transactions , 2002, vol. 33A, pp. 2185-2194.
47. Y. Wu, R. M. German, D. Blaine, B. Marx, C. Schlaefer, “Effects of Residual Carbon Content on Sintering Shrinkage, Microstructure and Mechanical Properties of Injection Molded 17-4 PH Stainless Steel,” Journal of Materials Science , 2002, vol. 37, pp. 3573-3583.
48. J. Stampfl, H. C. Liu, S. W. Nam, K. Sakamoto, H. Tsuru, S. Kang, A. G. Cooper, A. Nckel, F. B Prinz, “Rapid prototying and manufacturing by gelcasting of metallic and ceramic slurries,” Materials Science and Engineering , 2002, vol. A334, pp. 187-192.
49. M. K. Bulger, A. R. Erickson, “Fatigue Properties of MIM Fe-7% Ni and 17-4 PH,” Powder Injection Molding Symposium 1992 , P. H. Booker, J. Gaspervich and R. M. German (eds.), Metal Powder Industries Federation, Princeton, NJ, 1992, pp. 493-508.
50. T. Findik, S. Tasdemir, I. Sahin, “The use of artificial neural network for prediction of grain size in 17-4 PH stainless steel powders,” Scientific Research and Essays , 2010, vol. 5, pp. 1274-1283.
51. R. M. German, D. Kubish, “Evaluation of Injection Molded 17-4 PH Stainless Steel Using Water Atomized Powder,” International Journal of Powder Metallurgy , 1993, vol. 29, pp. 47-62.
52. R. M. Schmees, J. J. Valencia, “Mechanical Properties of Powder Injection Molded Inconel 718,” Advances in Powder Metallurgy and Particulate Materials - 1998 , Metal Powder Industries Federation, Princeton, NJ, 1998, pp. 5.107-5.118.
53. H. O. Gulsoy, S. Ozbek, T. Baykara, “Microstructural and Mechanical Properties of Injection Moulded Gas and Water Atomized 17-4 PH Stainless Steel Powder,” Powder Metallurgy , 2007, vol. 50, pp. 120-126.
54. D. C. Blaine, S. J. Park, R. M. German, J. Lasalle, H. Nandi, “Verifying the Master Sintering Curve on an Industrial Furnace,” Advances in Powder Metallurgy and Particulate Materials - 2005 , Metal Powder Industries Federation, Princeton, NJ, 2005, pp. 1.13-1.19.
55. R. T. Fox, D. Lee, M. K. Bulger, R. M. German, “Evaluation of Injection Molded Stainless Steel Powders,” Advances in Powder Metallurgy , vol. 3, Metal Powder Industries Federation, Princeton, NJ, 1990, pp. 359-373.
56. K. Murray, A. J. Coleman, T. A. Tingskog, D. T. Whychell, “Effect of Particle Size Distribution on Processing and Properties of MIM 17-4 PH,” Advances in Powder Metallurgy and Particulate Materials - 2010 , Metal Powder Industries Federation, Princeton, NJ, 2010, pp. 4.18-4.27.
57. B. Julien, M. Ste-Marie, R. Pelletier, F. Lapointe, S. Turenne, “Parametric Modeling of MIM As-Sintered Properties of Stainless Steel 17-4 PH and 316L Using a Statistical Model,” Advances in Powder Metallurgy and Particulate Materials - 2010 , Metal Powder Industries Federation, Princeton, NJ, 2010, pp. 4.1-4.10.
58. K. Murray, A. J. Coleman, T. Tingkskog, D. Whychell, “The Influence of Powder Size on Processing and Properties of MIM 17-4PH,” Proceedings PM 2010 World Congress , Florence Italy October 2010, European Powder Metallurgy Association, Shrewsbury, UK, on CD.
59. H. J. Sung, T. K. Ha, S. Ahn, Y. W. Chang, “Powder Injection Molding of a 17-4 PH Stainless Steel and the Effect of Sintering Temperature on its Microstructure and Mechanical Properties,” Journal of Materials Processing Technology , 2002, vol. 130, pp. 321-327.
60. H. O. Gulsoy, S. Salman, S. Ozbek, F. Findik, “Sintering of a Boron-Doped Injection Moulded 17-4 PH Stainless Steel,” Journal of Materials Science Letters , 2005, vol. 40, pp. 4101-4104.
61. C. W. Chang, P. H. Chen, K. S. Hwang, “Enhanced Mechanical Properties of Injection Molded 17-4 PH Stainless Steel Through Reduction of Silica Particles by Graphite Additions,” Materials Transactions , 2010, vol. 51, pp. 2243-2250.
62. P. Quirmbach, S. Schwartz, U. Dasbach, F. Petzoldt, L. Kramer, “Processing Parameters for a Continuous MIM-Production with an Aqueous Debinded 17-4 PH,” Advances in Powder Metallurgy and Particulate Materials - 2002 , Metal Powder Industries Federation, Princeton, NJ, 2002, pp. 10.284-10.294.
63. H. Irrinki, K. Kate, S. Atre, B. Kumar, J. Stitzel, S. Badwe, “Evaluation of Corrosion Properties of 17-4 PH Stainless Steel Processed by Laser-Powder Bed Fusion,” Advances in Powder Metallurgy and Particulate Materials – 2017 , Metal Powder Industries Federation, Princeton, NJ, 2017, on CD.
64. K. Murray, A. J. Coleman, T. Tingskog, D. T. Whychell, “Effect of Particle Size Distribution on Processing and Properties of 17-4 PH,” International Journal of Powder Metallurgy, 2011, vol. 47, no. 4, pp. 21-28.
65. Y. Li, L. Li, K. A. Khalil, “Effect of Powder Loading on Metal Injection Molding Stainless Steels,” Journal of Materials Processing Technology , 2007, vol. 183, pp. 432-439.
66. K. A. Khalil, S. W. Kim, “Relationship between Binder Content and Mechanical Properties of 17-4 PH Stainless Steel Fabricated by PIM Process and Sintering,” Metals and Materials International , 2006, vol. 12, pp. 1010-106.
67. B. N. Mukund, B. Hausnerova, T. S. Shivashankar, “Development of 17-4 PH stainless steel bimodal powder injection molding feedstock with the help of interparticle spacing / lubricating liquid concept,” Powder Technology , 2015, vol. 283, pp. 24-31.
68. R. M. German, Powder Metallurgy and Particulate Materials Processing , Metal Powder Industries Federation, Princeton, NJ, 2005.
69. C. Terrisse, L. Nyborg, P. Bracconi, “Surface Reaction during Vacuum Sintering of 316L Stainless Steel Powder,” Proceedings 1998 PM World Congress , Granada Spain, European Powder Metallurgy Association, Shrewsbury, UK, December 1998, on CD.
70. S. Banerjee, C. J. Joens, “A Comparison of Techniques for Processing Powder Metal Injection Molded 17-4 PH Materials,” Advanced in Powder Metallurgy and Particulate Materials - 2008 , Metal Powder Industries Federation, Princeton, NJ, 2008, on CD.
71. P. K. Minuth, P. Kunert, D. Meinhardt, G. Veltl, L. Kramer, T. Hartwid, “Mechanical Properties of MIM Parts Produced from Mixtures of Stainless Steel 17-4 PH,” Competitive Advantages by Near-Net-Shape Manufacturing , H. D. Kunze (ed.), DGM Informationsgellschaft, Frankfurt, Frankfurt, Germany, 1997, pp. 303-308.
72. G. Kalin, E. Akdag, S. Ozbey, H. O. Gulsoy, “Electrochemical Behavior of Injection Molded Gas and Water Atomized 17-4PH Stainless Steel Powder,” Advances in Powder Metallurgy and Particulate Materials , Metal Powder Industries Federation, Princeton, NJ, 2017, pp. 364-373.
73. R. M. German, “The Sintering of 304L Stainless Steel Powder,” Metallurgical Transactions , 1976, vol. 7A, pp. 1879-1885.
74. T. Shimizu, K. Matsuzaki, T. Sano, “Rapid Prototyping of Metallic Parts by Green Machining,” Journal of the Japan Society for Powder and Powder Metallurgy , 2006, vol. 53, pp. 791-796.
75. E. Klar, P. K. Samal, Powder Metallurgy Stainless Steels , ASM International, Materials Park, OH, 2007.
76. S. Krug, S. Zachmann, “Influence of Sintering Conditions and Furnace Technology on Chemical and Mechanical Properties of Injection Moulded 316L,” Powder Injection Moulding International , 2009, vol. 3, no. 4, pp. 66-71.
77. A. Bose, D. Agrawal, R. J. Dowding, “Preliminary Investigations into Microwave Processing of Powder Injection Molded 17-4 PH Stainless Steel,” Advances in Powder Metallurgy and Particulate Materials - 2004 , Part 4, Metal Powder Industries Federation, Princeton, NJ, 2004, pp. 53-60.
78. R. M. German, Sintering Theory and Practice , John Wiley and Sons, New York, NY, 1996.
79. C. K. Tai, C. H. Liang, “Study of Powder Characteristics on Mechanical Properties of Metal Injection Molding (MIM) Product,” Advances in Powder Metallurgy and Particulate Materials - 2007 , Metal Powder Industries Federation, Princeton, NJ, 2007, pp. 4.28-4.37.
80. J. Kazior, A. Szewczyk-Nykiel, T. Pieczonka, M. Hebda, M. Nykiel, “Properties of Precipitation Hardening 17-4 PH Stainless Steel Manufactured by Powder Metallurgy Technology,” Advanced Materials Research , 2013, vol. 811, pp. 87-92.
81. S. H. Chung, Y. S. Kwon, P. Suri, N. Erhardt, D. C. Blaine, C. Schlaefer, R. M. German, “Sintering Simulation of Powder Injection Molded 17-4 PH Stainless Steel,” Proceedings Sintering 2003 , R. G. Cornwall, R. M. German, and G. L. Messing (eds.), Materials Research Institute, Pennsylvania State University, University Park, PA, 2003, on CD.
82. Y. S. Kwon, Y. Wu, P. Suri, R. M. German, “Simulation of the Sintering Densification and Shrinkage Behavior of Powder Injection Molded 17-4 PH Stainless Steel,” Metallurgical and Materials Transactions , 2004, vol. 35A, pp. 257-263.
83. A. Simchi, A. Rota, P. Imgrund, “An investigation on the sintering behavior of 316L and 17-4PH stainless steel powders for graded composites,” Materials Science and Engineering , 2006, vol. A424, pp. 282-289.
84. D. C. Blaine, Y. Wu, C. E. Schlaefer, B. Marx, R. M. German, “Sintering Shrinkage and Microstructure Evolution during Densification of a Martensitic Stainless Steel,” Proceedings Sintering 2003 , R. G. Cornwall, R. M. German, and G. L. Messing (eds), Materials Research Institute, Pennsylvania State University, University Park, PA, 2003, on CD.
85. D. Blaine, S. H. Chung, S. J. Park, P. Suri, R. M. German, “Finite Element Simulation of Sintering Shrinkage and Distortion in Large PIM Parts,” P/M Science and Technology Briefs , 2004, vol. 6, no. 2, pp. 13-18.
86. D. C. Blaine, R. M. German, “Sintering Simulation for PIM Stainless Steel,” Advances in Powder Metallurgy and Particulate Materials - 2002 , Metal Powder Industries Federation, Princeton, NJ, 2002, pp. 10.255-10.266.
87. P. G. E. Jerrard, L. Hao, K. E. Evans. “Experimental investigation into selective laser melting of austenitic and martensitic stainless steel powder mixtures.” Proceedings of the Institution of Mechanical Engineers, Part B: Journal of Engineering Manufacture , 2009, vol. 223, pp. 1409-1416.
88. Y. Li, K. A. Khalil, B. Huang, Metal Injection Molding of 17-4 PH Stainless Steel , National Key Laboratory for Powder Metallurgy, Central South University, Changsha, China, 2005.
89. D. C. Blaine, S. J. Park, R. M. German, “Master Sintering Curve for a Two-Phase Material,” Proceedings of the 4th International Conference on Science, Technology and Applications of Sintering , D. Bouvard (ed.), Institut National Polytechnique de Grenoble, Grenoble, France, 2005, pp. 264-267.
90. R. M. German, “Coordination number changes during powder densification,” Powder Technology , 2014, vol. 243, pp. 368-376.
91. X. Xu, W. Yi, R. M. German, “Densification and Strength Evolution in Solid-State Sintering Part I, Experimental Investigation,” Journal of Materials Science , 2002, vol. 37, pp. 567-575.
92. H. J. Sung, T. K. Ha, S. Ahn, Y. W. Chang, “An Investigation on Elongation - Porosity Relation in Sintered 17-4 PH Stainless Steel,” Materials Science Forum , 2007, vol. 534, pp. 645-648.
93. H. Miura, T. Baba, “Sintering High Strength Injection Molded Stainless Steel Compacts,” Recent Progress in Iron Powder Metallurgy , R. Watanabe and K. Ogura (eds.), School of Engineering, Tohoku University, Sendai, Japan, 1999, pp. 47-57.
94. P. H. Chen, K. S. Hwang, “Mechanical Properties of Powder Injection Moulded 17-4 PH Sintered in Dissociated Ammonia,” Proceedings PM 2010 World Congress , Florence Italy October 2010, European Powder Metallurgy Association, Shrewsbury, UK, on CD.
95. T. Baba, H. Miura, T. Honda, Y. Tokuyama, “High Performance Properties of Injection Molded 17-4 PH Stainless Steel,” Advances in Powder Metallurgy and Particulate Materials - 1995 , Metal Powder Industries Federation, Princeton, NJ, 1995, pp. 6.271-6.278.
96. R. M. German, Powder Metallurgy of Iron and Steel , Wiley, Hoboken, NJ, 1998.
97. T. Baba, H. Miura, T. Honda, Y. Tokuyama, “Properties of 17-4 PH Stainless Steels Produced by Metal MIM 17-4 PH stainless steel Injection Molding Process,” Journal of the Japan Society of Powder and Powder Metallurgy , 1995, vol. 42, pp. 1119-1123.
98. R. M. F. Jones, “The Effect of Processing Variables on the Properties of 316L Powder Compacts,” Progress in Powder Metallurgy , 1974, vol. 30, pp. 25-50.
99. K. Kamada, M. Nakamura, H. Horie, “Development of High Strength P/M SUS 630 Stainless Steels Utilized by the Liquid Phase Sintering,” Proceedings of the 2000 Powder Metallurgy World Congress , Part 2, K. Kosuge and H. Nagai (eds.), Japan Society of Powder and Powder Metallurgy, Kyoto, Japan, 2000, pp. 1021-1024.
100. K. Kamada, M. Nakamura, H. Horie, “Effect of Addition of Boron and Silicon on Mechanical Properties of P/M SUS630 Stainless Steel,” Journal of the Japan Society of Powder and Powder Metallurgy , 2001, vol. 48, pp. 810-815.
101. H. O. Gulsoy, S. Salman, S. Ozbek, “Effect of FeB Additions on Sintering Characteristics of Injection Moulded 17-4 PH Stainless Steel Powder,” Journal of Materials Science , 2004, vol. 39, pp. 4835-4840.
102. H. O. Gulsoy, S. Ozbek, S. Salman, “Sintering and Mechanical Properties of Injection Molded 17-4 PH Stainless Steel Powder with FeB Additions,” Fourth International Powder Metallurgy Conference, Turkish Powder Metallurgy Association , Sakarya University, Sakarya, Turkey, 2005, pp. 415-428.
103. H. O. Gulsoy, S. Salman, “Microstructure and Mechanical Properties of Injection Molded 17-4 PH Stainless Steel Powder with Nickel Boride Additions,” Journal of Materials Science , 2005, vol. 40, pp. 3415-3421.
104. A. Szewczyk-Nykiel, The Effect of the Addition of Boron on the Densification, Microstructure, and Properties of Sintered 17-4 PH Stainless Steel , Institute of Materials Engineering, Cracow University of Technology, Cracow, Poland, 2014.
105. H. O. Gulsoy, “Influence of Nickel Boride Additions on Sintering Behaviors of Injection Moulded 17-4 PH Stainless Steel Powder,” Scripta Materialia , 2005, vol. 52, pp. 187-192.
106. B. Suharno, D. Ferdian, H. R. Saputro, L. P. Suharno, E. R. Baek, S. Supriadi, “Vacuum Sintering Process in Metal Injection Molding for 17-4 PH Stainless Steel as Material for Orthodontic Bracket,” Solid State Phenomena , 2017, vol. 266, pp. 231-237.
107. G. A. Shoales, R. M. German, “Combined Effects of Time and Temperature on Strength Evolution Using Integral Work-of-Sintering Concepts,” Metallurgical and Materials Transactions , 1999, vol. 30A, pp. 465-470.
108. S. V. Atre, G. A. Shoales, A. Lal, K. K. Comstock, D. M. Errick, R. M. German, “Strength Evolution During the Thermal De-binding of PIM Components,” Powder Injection Molding Technologies , R. M. German, H. Wiesner, and R. G. Cornwall (eds.), Innovative Material Solutions, State College, PA, 1998, pp. 217-226.
109. X. Xu, P. Lu, R. M. German, “Densification and Strength Evolution in Solid-State Sintering: Part II, Strength Model,” Journal of Materials Science , 2002, vol. 37, pp. 117-126.
110. R. Schroeder, G. Hammes, C. Binder, A. N. Klein, “Plasma De-binding and Sintering of Metal INjection Moulded 17-4 PH Stainless Steel,” Materials Research , 2011, vol. 14, pp. 564-568.
111. I. D. Jung, S. Ha, S. J. Park, D. C. Blaine, R. Bollina, R. M. German, “Two-Phase Master Sintering Curve for 17-4 PH Stainless Steel,” Metallurgical and Materials Transactions , 2016, vol. 47A, pp. 5548-5556.
112. J. J. Valencia, T. J. McCabe, H. Dong, “Microstructure and Mechanical Properties of Powder Injection Molded 17-4 PH Stainless Steel for Aircraft Engine Components,” Advances in Powder Metallurgy and Particulate Materials - 1995 , Metal Powder Industries Federation, Princeton, NJ, 1995, pp. 6.205-6.214.
113. J. L. LaGoy, M. K. Bulger, “Effect of HIP on the Microstructure and Impact Strength of MIM 17-4 PH Stainless Steel,” Advances in Powder Metallurgy and Particulate Materials - 2009 , Metal Powder Industries Federation, Princeton, NJ, 2009, pp. 4.70-4.80.
114. MatWeb Material Property Data, www.matweb.com.
115. M. Gross, “MIM helps Raytheon improve product performance and lower manufacturing costs,” Powder Injection Moulding International , 2014, vol. 8, no. 1, pp. 13-15.
116. H. Nakayama, H. Kyogoku, S. Komatsu, “Effect of Heat Treatment Conditions on Microstructure and Mechanical Properties of Sintered SUS630 Compacts by MIM Process,” J ournal of the Japan Society of Powder and Powder Metallurgy , 1998, vol. 45, pp. 882-886.
117. H. O. Gulsoy, R. M. German, “Sintered Foams from Precipitation Hardened Stainless Steel Powder,” Powder Metallurgy , 2008, vol. 51, pp. 350-353.
118. R. M. German, “Sintering Trajectories: Description on How Density, Surface Area, and Grain Size Change,” Journal of Metals (JOM) , 2016, vo. 68, pp. 878-884.
119. J. L. Johnson, A ssessment of the Properties of AMT Stainless Steels and Tool Steels , Technical Report #2003-05, AMTellect, State College, Pennsylvania, 2003.
120. P. Suri, B. P. Smarslok, R. M. German, “Impact Properties of Sintered and Wrought 17-4 PH Stainless Steel,” Powder Metallurgy , 2006, vol. 49, pp. 40-47.
121. J. A. Sago, M. W. Broadley, J. K. Eckert, R. J. White, “The Influence of Microstructure on the Mechanical Properties of 17-4 PH Stainless Steel,” Advances in Powder Metallurgy and Particulate Materials - 2007 , Metal Powder Industries Federation, Princeton, NJ, 2007, pp. 4.99-4.109.
122. S. Cheruvathur, E. A. Lass, C. E. Campbell, “Additive Manufacturing of 17-4 PH Stainless Steel: Post Processing Heat Treatment to Achieve Uniform Reproducible Microstructure,” Journal of Metals (JOM) , 2015, vol. 68, no. 3, pp. 930-942.
123. A. Ziewiec, A. Zielinska-Lipiec, E. Tasak, “Microstructure of Welded Joints of X5CrNiCuNb16-4 (17-4 PH) Martensitic Stainless Steel After Heat Treatment,” Archives of Metallurgy and Materials , 2014, vol. 59, pp. 965-970.
124. H. Miura, T. Baba, “High Performance Injection Molded Stainless Steels,” Processing and Fabrication of Advanced Materials VI , vol. 2, K. A. Khor, T. S. Srivatsan, J. J. Moore (eds.), Institute of Materials, London, UK, 1998, pp. 1377-1387.
125. A. Gratton, “Comparison of Mechanical, Metallurgical Properties of 17-4 PH Stainless Steel between Direct Metal Laser Sintering (DMLS) and Traditional Manufacturing Methods,” Proceedings of the National Conference on Undergraduate Research , Weber State University, Ogden, UT, 2012.
126. C. J. Joens, “Laminar Gas Flow Enhances Debind and Sinter of PIM Parts,” Industrial Heating , 2004, June, pp. 37-39.
127. J. L. Johnson, “Corrosion Resistant Medical Instruments Produced by Metal Injection Molding,” Medical Device Materials , S. Shrivastave (ed.), ASM International, Materials Park, OH, 2004, pp. 408-413.
128. H. J. Rack, D. Kalish, “The Strength, Fracture Toughness, and Low Cycle Fatigue Behavior of 17-4 PH Stainless Steel,” Metallurgical Transactions , 1974, vol. 5, pp. 1595-1605.
129. R. M. German, Particulate Composites , Springer, Basel Switzerland, 2016, pp. 127-130.
130. S. A. Slaby, O. Kraft, C. Eberl, “Fatigue Properties of Conventionally Manufactured and Micro Powder Injection Moulded 17-4 PH Microcomponents,” Fatigue and Fracture of Engineering Materials and Structures , 2016, vol. 39, pp. 780-789.
131. R. M. German, Metal Injection Molding A Comprehensive MIM Design Guide , Metal Powder Industries Federation, Princeton, NJ, 2011.
132. P. K. Samal, N. Nandivada, I. Hauer, “Properties of 17-4 PH Stainless Steel Produced via Press and Sinter Route,” Advances in Powder Metallurgy and Particulate Materials - 2008 , Metal Powder Industries Federation, Princeton, NJ, 2008, Part 7, pp. 109-120.
133. S. Bannerjee, “Structure - Property Relationship of Metal Injection Molded 17-4 PH Orthodontic Parts,” Powder Injection Molding Symposium 1992 , P. H. Booker, J. Gaspervich and R. M. German (eds.), Metal Powder Industries Federation, Princeton, NJ, 1992, pp. 181-192.
134. H. Wohlfromm, M. Blomacher, P. J. Uggowitzer, R. Magdowski, M. O. Speidel, “Corrosion Resistance of MIM Stainless Steels,” Advanced in Powder Metallurgy and Particulate Materials – 1999 , vol. 2, Metal Powder Industries Federation, Princeton, NJ, 1999, pp. 6.27-6.38.
135. I. Costa, C. V. Franco, C. T. Kunioshi, J. L. Rossi, “Corrosion Resistance of Injection Molded 17-4PH Steel in Sodium Chloride Solution,” Corrosion , 2006, vol. 62, pp. 357-365.
136. A. Szewczyk-Nykiel, J. Kazior, “Effect of Aging Temperature on Corrosion Behavior of Sintered 17-4 PH Stainless Steel in Dilute Sulfuric Acid Solution,” J ournal of Materials Engineering and Performance , 2017, vol. 26, pp. 3450-3456.
137. T. Oh, S. U. Choo, K. M. Kim, K. N. Kim, “A stainless steel bracket for orthodontic application,” European Journal of Orthodontics , 2005, vol. 27, pp. 237-244.
138. J. A. Sago, M. W. Broadley, J. K. Eckert, H. Chen, “Manufacturing of Implantable Biomedical Devices by Metal Injection Molding,” Advances in Powder Metallurgy and Particulate Materials - 2010 , Metal Powder Industries Federation, Princeton, NJ, 2010, pp. 4.89-4.99.
139. R. M. German, “Green Body Homogeneity Effects on Sintered Tolerances,” Powder Metallurgy , 2004, vol. 47, pp. 157-160.
140. D. C. Blaine, S. J. Park, P. Suri, R. M. German, “Application of Work of Sintering Concepts in Powder Metallurgy,” Metallurgical and Materials Transactions , 2006, vol. 37A, pp. 2827-2835.
作者简介:
邱耀弘,毕业于台湾科技大学,机械工程系博士,现任东莞理工学院长安先进制造学院助理教授、中国MIM产业顾问、中国数家企业项目顾问。专长氢燃料电池总成开发、无机粉末注射成形、机械设计与机械制造、材料与破坏分析科学、气相物理沈积,发表有关技术文章超过20篇。多次受邀参加国际和国内大型会议演讲,“无私分享,升级产业”,被天下杂志评论为黑手博士。
本文已获邱耀弘授权发布
